Copper(I) sulfide
| |
| Names | |
|---|---|
| IUPAC name
Copper(I) sulfide
| |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.040.751 |
PubChem CID
|
|
| RTECS number |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Cu2S | |
| Molar mass | 159.16 g/mol |
| Density | 5.6 g/cm3 [1] |
| Melting point | 1,130 °C (2,070 °F; 1,400 K)[2] |
| Insoluble | |
| Solubility | slightly soluble in HCl; soluble in NH4OH; dissolves in KCN; decomposes in HNO3, H2SO4 |
| Hazards | |
| Flash point | Non-flammable |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 1 mg/m3 (as Cu)[3] |
REL (Recommended)
|
TWA 1 mg/m3 (as Cu)[3] |
IDLH (Immediate danger)
|
TWA 100 mg/m3 (as Cu)[3] |
| Related compounds | |
Other anions
|
Copper(I) oxide Copper(I) selenide |
Other cations
|
Nickel(II) sulfide Copper(II) sulfide Zinc sulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Copper(I) sulfide is a copper sulfide, a chemical compound of copper and sulfur. It has the chemical compound Cu2S. It is found in nature as the mineral chalcocite. It has a narrow range of stoichiometry ranging from Cu1.997S to Cu2.000S.[4]
Preparation and reactions
Cu2S can be prepared by heating copper strongly in sulfur vapour or H2S.[2] The reaction of copper powder in molten sulfur rapidly produces Cu2S, whereas pellets of copper require much higher temperature.[5] Cu2S reacts with oxygen to form SO2:[6]
- 2 Cu2S + 3 O2 → 2 Cu2O + 2 SO2
In the production of copper two thirds of the molten copper sulfide is oxidised as above, and the Cu2O reacts with unoxidised Cu2S to give Cu metal:[6]
- Cu2S + 2 Cu2O → 6 Cu + SO2
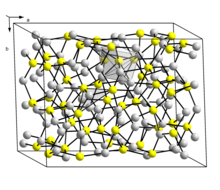
Structure
There are two forms of Cu2S: a low temperature monoclinic form ("low-chalcocite") which has a complex structure with 96 copper atoms in the unit cell[7] and a hexagonal form stable above 104 °C.[8] In this structure there are 24 crystallographically distinct Cu atoms and the structure has been described as approximating to a hexagonal close packed array of sulfur atoms with Cu atoms in planar 3 coordination. This structure was initially assigned an orthorhombic cell due to the twinning of the sample crystal.
There is also a crystallographically-distinct phase (the mineral djurleite) with stoichiometry Cu1.96S which is non-stoichiometric (range Cu1.934S-Cu1.965S) and has a monoclinic structure with 248 copper and 128 sulfur atoms in the unit cell.[7] Cu2S and Cu1.96S are similar in appearance and hard to distinguish one from another.[9]
See also
- copper sulfide for an overview of all copper sulfide phases
- copper monosulfide, CuS
- Chalcocite
- Djurleite
References
- ^ Patnaik, Pradyot (2002). Handbook of Inorganic Chemicals. McGraw-Hill, ISBN 0-07-049439-8
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. p. 1373. ISBN 978-0-08-022057-4.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0150". National Institute for Occupational Safety and Health (NIOSH).
- ^ Potter, R. W. (1977). "An electrochemical investigation of the system copper-sulfur". Economic Geology. 72 (8): 1524–1542. doi:10.2113/gsecongeo.72.8.1524.
- ^ Blachnik R., Müller A. (2000). "The formation of Cu2S from the elements I. Copper used in form of powders". Thermochimica Acta. 361: 31. doi:10.1016/S0040-6031(00)00545-1.
- ^ a b Wiberg, Egon and Holleman, Arnold Frederick (2001) Inorganic Chemistry, Elsevier ISBN 0-12-352651-5
- ^ a b Evans, H. T. (1979). "Djurleite (Cu1.94S) and Low Chalcocite (Cu2S): New Crystal Structure Studies". Science. 203 (4378): 356–8. doi:10.1126/science.203.4378.356. PMID 17772445.
- ^ Wells A.F. (1984) Structural Inorganic Chemistry, 5th ed., Oxford Science Publications, ISBN 0-19-855370-6
- ^ Evans H.T. (1981). "Copper coordination in low chalcocite and djurleite and other copper-rich sulfides" (PDF). American Mineralogist. 66 (7–8): 807–818.
