Toxoplasma gondii
| Toxoplasma gondii | |
|---|---|
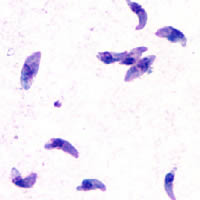
| |
| T. gondii tachyzoites | |
| Scientific classification | |
| Domain: | |
| Kingdom: | |
| Superphylum: | |
| Phylum: | |
| Class: | |
| Subclass: | |
| Order: | |
| Family: | |
| Subfamily: | |
| Genus: | Toxoplasma
|
| Species: | T. gondii
|
| Binomial name | |
| Toxoplasma gondii (Nicolle & Manceaux, 1908)
| |
Toxoplasma gondii is a species of parasitic protozoa in the genus Toxoplasma.[1]
The definitive host of T. gondii is the cat, but the parasite can be carried by many warm-blooded animals (birds[2] or mammals, including humans). Toxoplasmosis, the disease of which T. gondii is the causative agent, is usually minor and self-limiting but can have serious or even fatal effects on a fetus whose mother first contracts the disease during pregnancy or on an immunocompromised human or cat.
Life cycle

The life cycle of T. gondii has two phases. The sexual part of the life cycle (coccidia like) takes place only in cats, both domestic and wild (family Felidae),[3] which makes cats the parasite's primary host. The second phase, the asexual part of the life cycle, can take place in other warm-blooded animals, including cats, mice, humans, and birds. The hosts in which asexual reproduction takes place is called the intermediate host. Rodents are the typical intermediate host.[3]

In both kinds of hosts, the Toxoplasma parasite invades cells and forms a space called a vacuole. Inside this specialized vacuole, called a parasitophorous vacuole, the parasite forms bradyzoites, which are the slowly replicating versions of the parasite.[4] The vacuoles containing the reproductive bradyzoites form cysts mainly in the tissues of the muscles and brain. Since the parasites are inside cells, they are safe from the host's immune system, which does not respond to the cysts.[citation needed]
Toxoplasma's resistance to anti-toxoplasmosis medication varies, but the cysts are very difficult to eradicate entirely. Inside the vacuoles, T. gondii replicates itself (by endodyogeny) until the infected cell fills with parasites and bursts, releasing tachyzoites, the motile, asexually reproducing form of the parasite. Unlike the bradyzoites, the free tachyzoites are usually efficiently cleared by the host's immune system, although some of them manage to infect cells and form bradyzoites, thus maintaining the infection.[citation needed]
Tissue cysts are ingested by a cat (e.g., by feeding on an infected mouse). The cysts survive passage through the stomach of the cat and the parasites infect epithelium of the small intestine where they undergo sexual reproduction and oocyst formation. Oocysts are shed with the feces. Animals and humans that ingest oocysts (e.g., by eating unwashed vegetables) or tissue cysts in improperly cooked meat become infected. The parasite enters macrophages in the intestinal lining and is distributed via the blood stream throughout the body.[citation needed]
Similar to the mechanism used in many viruses, Toxoplasma is able to dysregulate host’s cell cycle by holding cell division before mitosis (the G2/M border).[5] This dysregulation of the host’s cell cycle is caused by a heat-sensitive secretion (with a molecular mass larger than 10 kDa).[6] Infected cells secrete the factor which inhibits the cell cycle of neighboring cells. The reason for Toxoplasma’s dysregulation is unknown, but studies have shown that infection is preferential to host cells in the S-phase and host cell structures with which Toxoplasma interacts may not be accessible during other stages of the cell cycle.[7][8][9][10][11]
Acute stage Toxoplasma infections can be asymptomatic, but often give flu-like symptoms in the early acute stages, and like flu can become, in very rare cases, fatal. The acute stage fades in a few days to months, leading to the latent stage. Latent infection is normally asymptomatic; however, in the case of immunocompromised patients (such as those infected with HIV or transplant recipients on immunosuppressive therapy), toxoplasmosis can develop. The most notable manifestation of toxoplasmosis in immunocompromised patients is toxoplasmic encephalitis, which can be deadly. If infection with T. gondii occurs for the first time during pregnancy, during an activity such as changing cat litter of a cat infected with T. gondii (uptake of cyst by inhalation, followed by ingestion as the mucus is cleared), the parasite can cross the placenta, possibly leading to hydrocephalus or microcephaly, intracranial calcification, and chorioretinitis, with the possibility of spontaneous abortion (miscarriage) or intrauterine death.[citation needed]
An in vitro study showed that ivermectin significantly inhibited T. gondii replication[12][13].
Epidemiology
The rates of positive sero-prevalence in women at child-bearing age between 1990 and 2000 were 58% in Central European countries, 51–72% in several Latin-American countries and 54–77% in West African countries. Low seroprevalence, 4–39%, was reported in southwest Asia, China and Korea as well as in cold climate areas such as Scandinavian countries (11–28%).[14]
T. gondii has also been linked to pre-natal depression, as well as increased anxiety and depression during pregnancies. It has also been linked with mood disturbances in nonpregnant populations,[15] including schizophrenia and suicidal behavior.[16]
Toxoplasmosis

T. gondii infections have the ability to change the behavior of rats and mice, making them drawn to, rather than fearful of, the scent of cats. This effect is advantageous to the parasite, which will be able to sexually reproduce if its host is eaten by a cat.[17] The infection is widespread in the brain, with more cysts targeting the parts of the brain corresponding to fear. The widespread nature of the infection causes many previously unnoticed symptoms in the rats.[18]
Possible benefits ? It is unclear, however, if in cases of dopamine deficiency disorders such as in many cases of ADD and ADHD, Toxoplasma gondii can actually improve cognitive performance. Such improvement can be detected in cognitive load tests which involves testing the Working Memory. The ability of Toxoplasma gondii to elevate dopamine levels may be detrimental to individuals whose brain has excess in dopamine levels such as to Schizophrenia patients. In ordinary people, enhancement in dopamine levels may cause extra brain connections and may lead to less neural efficiency. Synaptic pruning minimizes metabolic costs [A neuro-computational account of taxonomic responding and fast mapping in early word learning ,Julien Mayor & Kim Plunkett]. This pruning is reduced when Toxoplasma gondii is present. Pruning may also be important for fast response times. High metabolic cost means more glial cells biochemical production and better nourishment of the parasite by this biochemical production. This shows another motivation for the parasite to elevate dopamine levels in the brain. In individuals with dopamine deficiency, however, Toxoplasma gondii may be beneficial. Such individuals suffer from organization problems and from low Working Memory performance, e.g. they often forget objects when a new task captures their attention. In those individuals, it is possible that Toxoplasma gondii infection improves cognitive performance and reduce response time !!! It is also unclear if Toxoplasma gondii offers a natural cure for people with anxiety disorders. The reason is that the Amygdala, which processes fears, loses some of the fears when infected. In addition, dopamine level is responsible for optimism and thus depressed individuals may also benefit from Toxoplasma gondii infection. [Tali Sharot, Marc Guitart-Masip,Christoph W. Korn, Rumana Chowdhury,Raymond J. Dolan, How Dopamine Enhances an Optimism Bias in Humans].
Studies have also shown behavioral changes in humans, including lower reaction times and a sixfold increased risk of traffic accidents among infected, RhD-negative males,[19] as well as links to schizophrenia including hallucinations and reckless behavior. Recent epidemiologic studies by Stanley Medical Research Institute and Johns Hopkins University Medical Center indicate that infectious agents may contribute to some cases of schizophrenia.[20][21] A study of 191 young women in 1999 reported higher intelligence and higher guilt proneness in Toxoplasma-positive subjects.[22]
The prevalence of human infection by Toxoplasma varies greatly between countries. Factors that influence infection rates include diet (prevalence is possibly higher where there is a preference for less-cooked meat) and proximity to cats.[23]
According to Merck the standard treatment for toxoplasmosis is pyrimethamine, but most immunocompetent asymptomatic people infected with T. gondii, with the exception of neonates and pregnant women, require no treatment.[24]
History
The organism was first described in 1908 in Tunis by Charles Nicolle and Louis Manceaux within the tissues of the gundi (Ctenodactylus gundi). In the same year it was also described in Brazil by Alfonso Splendore in rabbits.
References
- ^ Ryan KJ, Ray CG (eds) (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. pp. 722–7. ISBN 0-8385-8529-9.
{{cite book}}:|author=has generic name (help) - ^ Dubey JP, Webb DM, Sundar N, Velmurugan GV, Bandini LA, Kwok OC, Su C. (2007-09-30). "Endemic avian toxoplasmosis on a farm in Illinois: clinical disease, diagnosis, biologic and genetic characteristics of Toxoplasma gondii isolates from chickens (Gallus domesticus), and a goose (Anser anser)". Vet Parasitol. 148 (3–4): 207–12. doi:10.1016/j.vetpar.2007.06.033. PMID 17656021.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Boothroyd JC (2009). "Toxoplasma gondii: 25 years and 25 major advances for the field". International Journal for Parasitology. 39 (8): 935–46. doi:10.1016/j.ijpara.2009.02.003. PMC 2895946. PMID 19630140.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Dubey, JP; Lindsay, DS; Speer, CA (1998). "Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts". Clinical Microbiology Reviews. 11 (2): 267–99. PMC 106833. PMID 9564564.
- ^ Blader, IRA J.; Saeij, Jeroen P. (2009). "Communication betweenToxoplasma gondiiand its host: Impact on parasite growth, development, immune evasion, and virulence". APMIS. 117 (5–6): 458–76. doi:10.1111/j.1600-0463.2009.02453.x. PMC 2810527. PMID 19400868.
- ^ Lavine, MD; Arrizabalaga, G (2008). "Exit from host cells by the pathogenic parasite Toxoplasma gondii does not require motility". Eukaryotic cell. 7 (1): 131–40. doi:10.1128/EC.00301-07. PMC 2224157. PMID 17993573.
- ^ Dvorak, J.; Crane, M. (1981). "Vertebrate cell cycle modulates infection by protozoan parasites". Science. 214 (4524): 1034–6. doi:10.1126/science.7029713. PMID 7029713.
- ^ Grimwood, J; Mineo, JR; Kasper, LH (1996). "Attachment of Toxoplasma gondii to host cells is host cell cycle dependent". Infection and immunity. 64 (10): 4099–104. PMC 174343. PMID 8926075.
- ^ Youn, J H; Nam, H W; Kim, D J; Park, Y M; Kim, W K; Kim, W S; Choi, W Y (1991). "Cell cycle-dependent entry of Toxoplasma gondii into synchronized HL-60 cells". The Korean Journal of Parasitology. 29 (2): 121–8. doi:10.3347/kjp.1991.29.2.121.
- ^ Coppens, Isabelle; Dunn, Joe Dan; Romano, Julia D.; Pypaert, Marc; Zhang, Hui; Boothroyd, John C.; Joiner, Keith A. (2006). "Toxoplasma gondii Sequesters Lysosomes from Mammalian Hosts in the Vacuolar Space". Cell. 125 (2): 261–74. doi:10.1016/j.cell.2006.01.056. PMID 16630815.
- ^ Walker, Margaret E.; Hjort, Elizabeth E.; Smith, Sherri S.; Tripathi, Abhishek; Hornick, Jessica E.; Hinchcliffe, Edward H.; Archer, William; Hager, Kristin M. (2008). "Toxoplasma gondii actively remodels the microtubule network in host cells". Microbes and Infection. 10 (14–15): 1440–9. doi:10.1016/j.micinf.2008.08.014. PMC 2765197. PMID 18983931.
- ^ "Invitro effects of ivermectin and sulphadiazine on Toxoplasma gondii" 20th European Congress of Clinical Microbiology and Infectious Diseases.
- ^ "Invitro effects of ivermectin and sulphadiazine on Toxoplasma gondii". 20th European Congress of Clinical Microbiology and Infectious Diseases. 2010.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Rorman, Efrat; Zamir, Chen Stein; Rilkis, Irena; Ben-David, Hilla (May 2006). "Congenital toxoplasmosis—prenatal aspects of Toxoplasma gondii infection". Reproductive Toxicology. 21 (4): 458–472. doi:10.1016/j.reprotox.2005.10.006.
{{cite journal}}:|access-date=requires|url=(help) - ^ Groër, Maureen W.; Yolken,, Robert H.; Xiao, J.-C.; Beckstead, Jason W.; Fuchs, Dietmar; Mohapatra, Shyam S.; Seyfang, Andreas; Postolache, Teodor T. (2011), "Prenatal depression and anxiety in Toxoplasma gondii–positive women", American Journal of Obstetrics and Gynecology, 204 (5): 433.e1-433.e7, doi:10.1016/j.ajog.2011.01.004
{{citation}}: Unknown parameter|month=ignored (help)CS1 maint: extra punctuation (link) - ^ Okusaga, Olaoluwa; Langenberg, Patricia; Sleemi, Aamar; Vaswani, Dipika; Giegling, Ina; Hartmann, Annette M.; Konte, Bettina; Friedl, Marion; Groer, Maureen W.; Yolken, Robert H.; Rujescu, Dan; Postolache, Teodor T. (2011), "Toxoplasma gondii antibody titers and history of suicide attempts in patients with schizophrenia", Schizophrenia Research, 133 (1–3): 150–155, doi:10.1016/j.schres.2011.08.006
{{citation}}: Unknown parameter|month=ignored (help) - ^ Berdoy M, Webster JP, Macdonald DW (2000). "Fatal attraction in rats infected with Toxoplasma gondii". Proc. Biol. Sci. 267 (1452): 1591–4. doi:10.1098/rspb.2000.1182. PMC 1690701. PMID 11007336.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ theatlantic.com
- ^ Flegr J, Klose J, Novotná M, Berenreitterová M, Havlíček J (2009). "Increased incidence of traffic accidents in Toxoplasma-infected military drivers and protective effect RhD molecule revealed by a large-scale prospective cohort study". BMC Infectious Diseases. 9: 72. doi:10.1186/1471-2334-9-72. PMC 2692860. PMID 19470165.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ cdc.gov
- ^ newscientist.com
- ^ Flegr J.; Havlícek J. (1999). Changes in the personality profile of young women with latent toxoplasmosis. Folia Parasitologica (Praha). 46(1):22-8. Note that the abstract misquotes the body text by reporting lower guilt proneness; findings, mentioned in several places in the text, were for high guilt proneness, not lower.
- ^ Meerburg BG, Kijlstra A (2009). "Changing climate—changing pathogens: Toxoplasma gondii in North-Western Europe". Parasitology Research. 105 (1): 17–24. doi:10.1007/s00436-009-1447-4. PMC 2695550. PMID 19418068.
- ^ Pearson, Richard D. (December 2009). "Toxoplasmosis". The Merck Manual. Merck Sharp & Dohme Corp. Retrieved 13 February 2012.
External links
- ToxoDB : The Toxoplasma gondii genome resource
- Anti-Toxo : A Toxoplasma news blog and list of research laboratories
- Toxoplasma images, from CDC's DPDx, in the public domain
- Toxoplasmosis Research Institute & Center
- Cytoskeletal Components of an Invasion Machine — The Apical Complex of Toxoplasma gondii
- The Culture-Shaping Parasites, in Seed Magazine
- Sneaky Parasite Attracts Rats to Cats, All Things Considered, April 14, 2007
- Toxoplasma overview, developmental stages, life cycle image at MetaPathogen
- Toxoplasma lecture, Robert Sapolsky
- [dead link] Could a brain parasite found in cats help soccer teams win at the World Cup? - By Patrick House - Slate Magazine
- How Your Cat Is Making You Crazy, the Atlantic Magazine, March 2012
- Mystery Marine Mammal Deaths, CosmosMagazine.com, June 2008
