Wikipedia:Reference desk/Science
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
December 29
ClF3 vs. (CF2)n
Can chlorine trifluoride oxidize polytetrafluoroethylene? Whoop whoop pull up Bitching Betty | Averted crashes 00:51, 29 December 2011 (UTC)
- The fact that both are used in uranium hexafluoride processing would suggest no. Rmhermen (talk) 01:39, 29 December 2011 (UTC)
- But PTFE contains carbon-carbon bonds, which ClF3 can break apart. Whoop whoop pull up Bitching Betty | Averted crashes 02:28, 29 December 2011 (UTC)
- CDC says Teflon is non-reactive with ClF3: [www.cdc.gov/niosh/docs/81-123/pdfs/0117.pdf] Rmhermen (talk) 04:25, 29 December 2011 (UTC)
- But PTFE contains carbon-carbon bonds, which ClF3 can break apart. Whoop whoop pull up Bitching Betty | Averted crashes 02:28, 29 December 2011 (UTC)
life
Well there are a lot of mind boggling questions about abiogenesis in my mind that I can't put it in words very accurately.But the one that I can say is the following: Is the simplest life form ever known to man irreducibly complex, according to current understanding of biology?And in what point could the structures be called life? Is there any scientific theory that states that life is a property of all matter(because I sometimes tend to think likewise!)?--Irrational number (talk) 13:11, 29 December 2011 (UTC)
- You can find some good minimum definitions of life here: life. --Goodbye Galaxy (talk) 15:09, 29 December 2011 (UTC)
- Some of these questions are tackled in "The origins of order" [1], or the more pop-sci version "At home in the universe" [2]. One of the key themes is that life-like structures are an expected, emergent property of complex systems. SemanticMantis (talk) 15:19, 29 December 2011 (UTC)
- According to the current understanding of Biology, no life is irreducibly complex in the sense of evolution being unable to produce it, though that understanding includes the realisation that biologists may not yet had the time to work out the details of all claimed instances of irreducible complexity. {The poster formerly known as 87.81.2301.95} 90.193.78.56 (talk) 15:26, 29 December 2011 (UTC)
- The first question is a bit too vague. "Irreducibly complex" is subjective to the human ability to replicate nature and is not synonymous with "impossible". We simply do not possess the technology required yet. Add to that the simple facts that Earth's conditions have changed drastically (ironically mostly as a result of the rise of life) and microscopic lifeforms almost always never leave recognizable fossils. So all life is basically irreducibly complex in terms of laboratory replication, but that does not automatically mean life is designed either (it's possible of course, but unlikely).
- The definition of life is also subjective and adds to the problem of the first question. Life is currently defined on what life we can find and recognize and makes little or no concessions as to possible or existing lifeforms that do not meet the requirements. As such, the "simplest" lifeforms (Pelagibacter ubique for free-living organisms and Mycoplasma genitalium for organisms dependent on other lifeforms for survival) are defined not because they are the most primitive, but because they have the smallest genomes for anything that meets the most widely accepted definition of "life". However, they are obviously "devolved" (or more accurately, stripped down for maximum efficiency) descendants of more complex earlier lifeforms and thus might not even resemble the precursors of life.
- Viruses may have also arisen similarly but are likewise generally not considered alive, as are organelles of possible endosymbiotic origins, etc. They are considered merely fragments of life. Hence the paradox that the earliest lifeforms would not actually be considered alive by today's definition, merely being chemicals that eventually gave rise to complex life by aggregation, endosymbiosis, horizontal gene transfer, assimilation, etc. with possibly widely different individual origins. Protobionts are examples of such "non-living" chemicals that may have given rise to life. And they have already been successfully synthesized in laboratory conditions mimicking probable early Earth environments.
- The problem is not that the lifeforms considered to be the simplest are too complex, it's that our lower limit for the definition of life is too high. It's like trying to find the origins of the car but excluding beast/human-drawn wagons (and wheels) because they are not self-propelled. Currently the border between life and the inanimate wavers somewhere around the protobiont nanobacteria and viruses.
- Lastly, I do not know of any such theories being seriously considered in science (maybe in metaphysics?) though I personally think attempts to restrict the definition of life is basically fruitless. It's a continuum, not an easily differentiated black-white distinction.-- Obsidi♠n Soul 15:27, 29 December 2011 (UTC)
Surely a major problem with this question on the Science Reference Desk is that it includes the term "irreducibly complex", a concept (almost?) exclusively used by creationists to prove science wrong. I don't think we should even be discussing this matter. Point out to the OP that irreducible complexity is a non-scientific concept, and move on. HiLo48 (talk) 22:46, 29 December 2011 (UTC)
- Criticisms of science are off-topic at the science refdesk? Really? --Trovatore (talk) 22:54, 29 December 2011 (UTC)
- Criticism based on fringe pseudo-science promoted by religiously driven nutcases? Yes. Off-topic. HiLo48 (talk) 04:44, 30 December 2011 (UTC)
- People who want to understand the scientific explanations for the origins of life should be encouraged, especially if they're trying to grapple with misinformation they've gotten in the past. Shunning someone who is willing to engage sounds like the worst plan for advancing science. Rckrone (talk) 05:58, 30 December 2011 (UTC)
- Some thoughts:
- 1) The idea of everything being alive is similar to the Gaia Hypothesis, which is more pseudoscience than true science.
- 2) Modern physics gives us the many worlds hypothesis, under which there may be an infinite number of universes. If true, then even the most unlikely events are bound to have occurred somewhere. And whoever evolves as a result may then look back at their evolution as an impossibility. StuRat (talk) 06:09, 30 December 2011 (UTC)
- That particular interpretation of the many worlds hypothesis sounds more like the Anthropic principle than the actual many worlds hypothesis. --Jayron32 06:12, 30 December 2011 (UTC)
- Okay, first, I'm sorry to use the word most scientists, and reasonable people, don't like.I'm an atheists myself, and according to what I know, the irreducible complexity is an argument (if it is at all) against evolution, not abiogenesis.but however, my question was "are there any species simpler than the first life form known to man?" which I kinda got a rough answer by reading the abiogenesis article, your answers and the links you provided.Thanks.-Irrational number (talk) 06:54, 30 December 2011 (UTC)
- and the Idea of life being a property of matter came to my mind from what Stephen Hawking once said:"life is just matter that has been given enough time."...--Irrational number (talk) 07:41, 30 December 2011 (UTC)
- For what it's worth, I call anything that can self-replicate "life". --Lgriot (talk) 09:12, 30 December 2011 (UTC)
- That list would include many things we don't normally think of as alive, like many chemicals, crystals, computer viruses, and even stars. StuRat (talk) 16:37, 30 December 2011 (UTC)
Natural phenomena after Kim Jong-il's death
What is the scientific explanation behind the strange phenomena occurred after his death? --Pakocat (talk) 14:47, 29 December 2011 (UTC)
- Propaganda. --Goodbye Galaxy (talk) 14:51, 29 December 2011 (UTC)
- Perhaps exploiting coincidence. {The poster formerly know as 87.81.2301.95} 90.193.78.56 (talk) 15:31, 29 December 2011 (UTC)
- = Propaganda and Confirmation bias.-- Obsidi♠n Soul 15:35, 29 December 2011 (UTC)
- For example: (1) Comet Lovejoy surviving perihelion; and (2) the Green Bay Packers losing their first game of the season. ←Baseball Bugs What's up, Doc? carrots→ 15:49, 29 December 2011 (UTC)
- Herd mentality, Groupthink, Deindividuation are a few more. Vespine (talk) 01:07, 30 December 2011 (UTC)
- For example: (1) Comet Lovejoy surviving perihelion; and (2) the Green Bay Packers losing their first game of the season. ←Baseball Bugs What's up, Doc? carrots→ 15:49, 29 December 2011 (UTC)
- = Propaganda and Confirmation bias.-- Obsidi♠n Soul 15:35, 29 December 2011 (UTC)
- Perhaps exploiting coincidence. {The poster formerly know as 87.81.2301.95} 90.193.78.56 (talk) 15:31, 29 December 2011 (UTC)
- It is only appropriate to hear of such reports since miracles also occurred on the day of his birth, when a new bright star shone in the heavens, a double rainbow appeared, and birds sang his praise in human voices.[3] Christopher Hitchens was fond of saying that North Korea was the most religious country he had ever visited. -- ToE 11:49, 31 December 2011 (UTC)
Skin regeneration
If one was to say cut a hand by glass with a flap of skin hanging would the skin then regenerate over a few weeks/months without stitches? If so what would it be normal or damaged? And how long does it take to regenerate? A search on the net said a month for youngsters with fresher skin, but a doc recently told me that it would not regenerate normally without stitches to hold it in place.Lihaas (talk) 20:01, 29 December 2011 (UTC)
- There is absolutely no way to answer this question. It totally depends on how deep the cut is, exactly where, what the cut is through, how much movement there is while it is healing, etc etc.. There's nothing really special about stitches, all they do is hold the wound closed tightly long enough for it to heal, but they do that extremely well, much better then just bandages. Vespine (talk) 22:01, 29 December 2011 (UTC)
- a flap of lose skin on the ring finger iright hand depper than the skin itself. a little turmeric was used to clean the wound, and really burnt. after the skin that was deadened for the stitching was reactivated it burnt (lack of blood after it was released, i guess).
- but what do you mean "how much movement"? in the sense that after the anaesthetic wore off there was a buroing sensation with a a sort of "heart beat" in the fingerLihaas (talk) 22:38, 29 December 2011 (UTC)
- I just mean how much movement the area of the wound is subject to. The main thing stitches do is create a mechanical brace which closes the wound, applies pressure and prevents the edges of the wound from moving during the healing process. Vespine (talk) 00:57, 30 December 2011 (UTC)
- And just to be sure, this is not an appropriate place to ask for medical advice, if you have any concern you need to go see a medical professional. Don't blame us if you lose a finger to infection. Vespine (talk) 01:03, 30 December 2011 (UTC)
- Not for me, some doc was telling me about a case and i did some research on the internet for a topic i knew nothing about.Lihaas (talk) 10:41, 30 December 2011 (UTC)
- And just to be sure, this is not an appropriate place to ask for medical advice, if you have any concern you need to go see a medical professional. Don't blame us if you lose a finger to infection. Vespine (talk) 01:03, 30 December 2011 (UTC)
- I just mean how much movement the area of the wound is subject to. The main thing stitches do is create a mechanical brace which closes the wound, applies pressure and prevents the edges of the wound from moving during the healing process. Vespine (talk) 00:57, 30 December 2011 (UTC)
Bedroom window
I have noticed that if I have my bedroom window open at night, to keep the bedroom cool and get fresh air, that I sleep much better. Is there more oxygen in cold air? Why does one sleep better with a open window at night? Dumb questions from a dumb blonde.--Christie the puppy lover (talk) 20:42, 29 December 2011 (UTC)
- The article on Circadian rhythm might help you. Human evolution means we have become accustomed to warm days and cold nights and so our circadian rhythm has adapted to that. why do blondes covet the title of 'dumb'? Some of my best friends are redheads and many take the biscuit in this attribute. --Aspro (talk) 20:56, 29 December 2011 (UTC)
- Ben Franklin already wrote about this: The Art of Procuring Pleasant Dreams. — Sebastian 03:50, 30 December 2011 (UTC)
- The temperature won't much affect the oxygen content, but a very well sealed house can have less oxygen inside, due to breathing and combustion (gas stoves, etc.). There could also be an accumulation of toxins inside, like carbon dioxide, hydrogen sulfide, and maybe even some carbon monoxide (which might put you to sleep permanently). StuRat (talk) 04:28, 30 December 2011 (UTC)
Thanks gentlemen. That helps out a dumb blonde alot.--Christie the puppy lover (talk) 00:00, 31 December 2011 (UTC)
- Gosh, we've be called gentleman. Must be due to that bottle of Braggi aftershave I was given at Christmas. --Aspro (talk) 00:07, 31 December 2011 (UTC)
Sugar Thermite?
I am curious if Aluminum would react with a sugar such as Glucose or Sucrose? Where the reaction removes Oxygen from the sugar and the products are a Carbon-Hydrgoen compound and Aluminum Oxide. Presuming the reaction even would occur. Similarly, could Calcium Oxide react with sugar to produce products of Calcium Carbonate and a Carbon-Hydrogen compound? I am more optimistic about the latter reaction, since if I was to combust the sugar with Oxygen first I would acquire water and Carbon Dioxide, which are used normally to create concrete from Calcium Oxide.Tartarean (talk) 21:16, 29 December 2011 (UTC)
- I don't know whether or not this could be done, but I'm sure you'd need some kind of catalyst: I'm confident that I could leave a Pepsi can in a vat of white sugar without anything dangerous happening. Nyttend (talk) 00:37, 30 December 2011 (UTC)
- I don't think it would happen, but not for the reasons Nyttend states. Thermite depends on finely powdered aluminum in order to work, you need a LOT of surface area. As far as a reaction of such finely powdered aluminum with sugar; I'm not so sure. Even if, thermodynaically, it should produce a product, it may not kinetically produce it at a rate to be considered proper "thermite". After all, rusting is technically a "combustion" reaction, at least in the sense that it describes a substance reacting with dioxygen. But no one would consider rusting to be anything like burning. Likewise, even if one were to replace the iron (iii) oxide normally present as the oxidizing agent in thermite with a carbon-based oxidizing agent, that wouldn't necessarily mean you'd get the spectacular thermite reaction. --Jayron32 00:55, 30 December 2011 (UTC)
- More basically, can sugar be used as an oxidant in a flashy, spectacular manner, in the way that iron oxide is in a thermite reaction? I can't find any examples, but I bet it can, under the proper circumstances. Magnesium is well known to burn in a carbon dioxide atmosphere or a chunk of dry ice. I bet if you encased a strip of Magnesium in sugar crystals, lit it up, and then flushed the atmosphere with Argon, it would continue to burn. No way of knowing without trying it though. Granted, Magnesium has a somewhat higher reduction potential than Aluminum. I know a couple people who have access to the required materials and safety equipment. Maybe after they get off break I'll try to talk them into an experiment. Buddy431 (talk) 06:38, 30 December 2011 (UTC)
- Sugar and sulfuric acid combine to make a fast-burning, gas-releasing mixture. Here's an explanation: The Dehydration of Sugar by Sulfuric Acid. Use caution: the reaction is very fast, very hot, releases a lot of steam - and I seem to recall it also produces some quantity of hydrogen sulfide gas, which can be toxic and explosive. Nonetheless, this sugar experiment is often performed in high school chemistry labs. Here's a video, Sugar Snake, showing the evolution into a oozing, charcoal-colored substance. I've performed this reaction and created a sugar snake that leaps up a lot faster - I may have had more concentrated acid than was used in the Youtube demo. (Comment: this is a dehydration, not a combustion, reaction).
- I have also seen sugar used as a (messy) rocket fuel. In pure Oxygen atmospheres, nearly anything will serve as a fuel; and sugar does have a lot of energy per unit mass. I don't think I've ever seen a sugar-like substance used as the oxidizer. Nimur (talk) 16:31, 30 December 2011 (UTC)
- Sure, sugar's used all the time as a fuel (reductant). Why do you consider it messy? Making Rocket candy has gotten down to almost a science in some amateur rocket circles. I've never heard of sugar as an oxidizer either, but I bet it can be done
- More basically, can sugar be used as an oxidant in a flashy, spectacular manner, in the way that iron oxide is in a thermite reaction? I can't find any examples, but I bet it can, under the proper circumstances. Magnesium is well known to burn in a carbon dioxide atmosphere or a chunk of dry ice. I bet if you encased a strip of Magnesium in sugar crystals, lit it up, and then flushed the atmosphere with Argon, it would continue to burn. No way of knowing without trying it though. Granted, Magnesium has a somewhat higher reduction potential than Aluminum. I know a couple people who have access to the required materials and safety equipment. Maybe after they get off break I'll try to talk them into an experiment. Buddy431 (talk) 06:38, 30 December 2011 (UTC)
- I don't think it would happen, but not for the reasons Nyttend states. Thermite depends on finely powdered aluminum in order to work, you need a LOT of surface area. As far as a reaction of such finely powdered aluminum with sugar; I'm not so sure. Even if, thermodynaically, it should produce a product, it may not kinetically produce it at a rate to be considered proper "thermite". After all, rusting is technically a "combustion" reaction, at least in the sense that it describes a substance reacting with dioxygen. But no one would consider rusting to be anything like burning. Likewise, even if one were to replace the iron (iii) oxide normally present as the oxidizing agent in thermite with a carbon-based oxidizing agent, that wouldn't necessarily mean you'd get the spectacular thermite reaction. --Jayron32 00:55, 30 December 2011 (UTC)
- So now that I'm actually awake I looked up some thermodynamic numbers. Using Red iron oxide and aluminum powder, the thermite reaction has a standard enthalpy of -425 kJ/mole Al. Using an idealized reaction of aluminum with Glucose, and giving a byproduct of 1-hexene (with enthalpy of formation taken from 1-Hexene (data page)), the standard enthalpy change is -541 kJ/mole Al, somewhat better than pure thermite. No, 1-hexene isn't likely to be the actual product in any significant amount, but it doesn't matter much: from a thermodynamic perspective, the reaction ought to take place.
- However, I've also been thinking about the reaction conditions. The byproduct, rather than iron, is going to be light hydrocarbons. While the iron formed simply flows down to the bottom of a thermite reaction (being more dense), these light hydrocarbons are going to vaporize as they're formed, potentially throwing flaming material everywhere. There's a good chance that they in turn would burn with the oxygen in the atmosphere, potentially leading to a pretty big fire hazard, more so than a normal metal oxide thermite reaction. If someone does try it, please be careful. Buddy431 (talk) 20:32, 30 December 2011 (UTC)
- As I mentioned above, thermodynamics is not the only factor in producing big flashy booms. Reaction kinetics are quite important as well, and determine the difference between a bang and a fizzle much more than thermodynamics will. --Jayron32 23:55, 30 December 2011 (UTC)
- However, I've also been thinking about the reaction conditions. The byproduct, rather than iron, is going to be light hydrocarbons. While the iron formed simply flows down to the bottom of a thermite reaction (being more dense), these light hydrocarbons are going to vaporize as they're formed, potentially throwing flaming material everywhere. There's a good chance that they in turn would burn with the oxygen in the atmosphere, potentially leading to a pretty big fire hazard, more so than a normal metal oxide thermite reaction. If someone does try it, please be careful. Buddy431 (talk) 20:32, 30 December 2011 (UTC)
- Bland question here on Ref Desk get deleted if there is any hint of them being medial but here is something that could be construed as aiding a would be terrorist. What are your priorities here???--Aspro (talk) 00:15, 31 December 2011 (UTC)
- A 'would-be terrorist' who has a large supply of finely-powdered aluminium and sugar, but no access to anything more conventional in the way of explosives? Admittedly some 'would-be terrorists' have demonstrated a remarkable level of incompetence, but even so... AndyTheGrump (talk) 00:47, 31 December 2011 (UTC)
- And like other people, thay may possess the ability to learn. Who would have thought... The chapati flour and bleach bomb. --Aspro (talk) 00:57, 31 December 2011 (UTC)
- Ok then, because terrorists might use information from Wikipedia, we should shut it down? And BTW, I think that even terrorists have the sense not to believe everything they read in the Daily Mail ;-) AndyTheGrump (talk) 05:09, 31 December 2011 (UTC)
- Great question. Thanks. The point I'm sarcastically making (all be it not very well) is that both clinicians and lawyers have succeeded in keeping their own discipline sacrosanct on Wikipedia. Why? To suggest meddling in their affairs is the most dangerous, is FUD in comparison the real world. Some of the advice regarding chemicals, engineering and other topics, make me glad that those posting the advice are not my next-door neighbors. So why on Wikipedia -that prides its self on neutrality- is this unexamined special respect, metered out on the subjects of law and medicine? Yet, if we question the Ref Desk posts such as this, it may help people to see this is a charade and an act of subtle but effective censorship. Can I take it also, that you have now tacitly admitted to actually having read a copy or two of the that wonderful edifier of the British hoi polloi entitled The Daily Mail? ;-) --Aspro (talk) 17:40, 31 December 2011 (UTC)
- Personally, I see discouraging unqualified people from giving medical and legal advice an act of common sense. If you want to argue otherwise, I suggest you raise the matter in a more appropriate place - though dragging out the same old tired clichés about 'censorship' isn't likely to get you far. AndyTheGrump (talk) 17:47, 31 December 2011 (UTC)
- This is the first time I've have brought up or heard mentioned this old tired cliché. It suggests to me some minds are fixed and petrified Telegraph readers.--Aspro (talk) 18:00, 31 December 2011 (UTC)
December 30
? on finding syndrome name
- The deletion can be seen here. Not to provide a diagnosis or anything, but "not being able to wear a wristwatch" is a common anecdote, but I can't find any actual studies on it. Though I could be wrong, I doubt you'd find any medical doctor to diagnose your condition, rural or otherwise. If you're looking for more information, of potentially dubious quality, you can just type "people who can't wear watches" into Google [4]. Buddy431 (talk) 05:45, 30 December 2011 (UTC)
- I can't wear a watch either (or, for that matter, glasses or any jewelry), but for different reasons. It does to my skin about what a cast does to anyone else's, turns it white and puffy. I had a pocket watch before cell phones came in, now I use that when I need to know the time. StuRat (talk) 05:53, 30 December 2011 (UTC)
Fractional Uncertainty with Zero Sloppiness
I'm trying to follow this and it starts out with a pool with the following measurements:
length L = 5.56 +/- 0.14 meters
= 5.56 m +/- 2.5%
I want to stop right there and note that the percentage having two significant figures makes sense since it would be calculated by ( 0.14 (2 sf) / 5.56 (3 sf) ) * 100% ( infinity sf)
width W = 3.12 +/- 0.08 meters
= 3.12 m +/- 2.6%
depth D = 2.94 +/m 0.11 meters
2.94 m +/- 3.7%
I think they got sloppy with the number of significant figures for the volume. Each of the length, width, and depth figures have 3 sfs, so the volume rounded to 3 sfs (let us suppose a round half to even convention in case we get a tie) is 51.0 m^3, but they put 51.00 m^3.
But that's not the thing that bothers me the most.
So the nominal measurements have 3 sfs, and the uncertainties have 2. As I already said, the fractional uncertainties should have 2 sfs. The convention they mention on that page is to add up the fractional uncertainties when measurements are multiplied or divided. Since a division was used to get fractional uncertainty, number of sfs was important, but now adding them, the lowest place value is what to look at. OK, adding 2.5% + 2.6% + 3.7% I get 8.8% which is kosher since each term is significant to the tenths place and so is the result. Now that we're about to multiply with that 8.8%, its number of sfs (2) should be what's important, right? Ignoring for a moment that they multiply their 51.00 * 8.8% (I would have multiplied it by 0.088, but I guess that's just notational), the bigger problem I see is that the uncertainty in volume should only have 2 sfs because the limiting figure was the 8.8% or 0.088 and not have an answer of 4.49 m^3 which has 3 (???) sfs (neither 4 like their nominal value nor 2 like their fractional uncertainty). I think the answer for nominal volume should be 51.0 m^3 with 3 sfs and volume uncertainty ought to be 51.0 m^3 * 0.088 = 4.5 m^3 with 2 sfs. This seems right because uncertainty is to the tenth of a cubic meter and the nominal volume is to the tenth of a cubic meter. Can anyone confirm they're wrong and I'm right or tell me why they're right, or what is right (with proof) if neither of us is? 69.243.220.115 (talk) 00:16, 30 December 2011 (UTC)
- Any use of significant figures is going to involve sloppiness. It can be a useful way of getting a rough and ready idea of the uncertainty in a number, but you are better off using the +/- numbers if they are known. If you don't want any sloppiness at all then don't convert the errors into percentages, keep them in the original form. --Tango (talk) 01:17, 30 December 2011 (UTC)
- If a professional engineer in the US as a member of ASME were given length 5.56 +/- 0.14 m, width 3.12 +/- 0.08 m, and depth 2.94 +/- 0.11 m, or a professional American physicist in the AIP were caring about this problem, and uncertainty in cubic meters were absolutely required (mentioning the original +/- numbers for each dimension were unacceptable, suppose the special concrete mix for the pool costs $100,000 per cubic meter, you only get one shot to pour it, and it's nonrefundable, nobody will buy your leftovers :) ), what would professional standards dictate is the correct answer? I know the problem at hand is with swimming pools, but with things like spacecraft and microchips, I doubt uncertainties are just considered 'rough and ready.' 69.243.220.115 (talk) 01:27, 30 December 2011 (UTC)
- See propagation of uncertainty for more details on this subject. The short answer is that there isn't an easy solution. You need to dig a little deeper into what those uncertainties really mean (ie. you need to know the probability distribution, or at least know whether it is reasonable to assume it is approximately normally distributed) and you need to know whether the uncertainties in the different measurements are connected at all (eg. if you underestimated the length, are you also likely to have underestimated the width?). If you have all of that information, then you can work it out. You can get some decent bounds on it using interval arithmetic, but that is only a little less rough and ready than significant figures. --Tango (talk) 03:15, 30 December 2011 (UTC)
- If a professional engineer in the US as a member of ASME were given length 5.56 +/- 0.14 m, width 3.12 +/- 0.08 m, and depth 2.94 +/- 0.11 m, or a professional American physicist in the AIP were caring about this problem, and uncertainty in cubic meters were absolutely required (mentioning the original +/- numbers for each dimension were unacceptable, suppose the special concrete mix for the pool costs $100,000 per cubic meter, you only get one shot to pour it, and it's nonrefundable, nobody will buy your leftovers :) ), what would professional standards dictate is the correct answer? I know the problem at hand is with swimming pools, but with things like spacecraft and microchips, I doubt uncertainties are just considered 'rough and ready.' 69.243.220.115 (talk) 01:27, 30 December 2011 (UTC)
- I agree with your criticism on excess significant figures, but we need to know what type of assumptions were made about the errors. If you are totally paranoid, and the errors are maximal rather than statistical, then the absolute maximum error is greater than 9% (range 55.632 to 46.629344 with unjustifiable accuracy!), but if the given errors are statistical then the maximum error in the volume is even greater, and the expected error smaller. We need to know the statistical model, as stated above. (The standard treatment assumes independent errors with a normal distribution.) You might be interested in this Wikipedia article: Experimental uncertainty analysis written by a retired nuclear scientist guy. Dbfirs 08:42, 30 December 2011 (UTC)
Pointed bullets
Why do guns fire blunt-nosed bullets instead of ones with sharp, pointed tips? Whoop whoop pull up Bitching Betty | Averted crashes 00:38, 30 December 2011 (UTC)
- Which guns and which bullets? There are all sorts of guns and all sorts of bullets, and they come in many shapes. --Jayron32 00:40, 30 December 2011 (UTC)
- Perhaps a little counter intuitively, in many cases a sharp pointed nose is not actually the most aerodynamically efficient design. We have an article Nose cone design which goes into some detail. Vespine (talk) 00:53, 30 December 2011 (UTC)
- See ballistics. Basically, the most streamlined shape for a supersonic projectile is one with a pointy nose, while the most streamlined shape for a subsonic projectile is one with a rounded nose. Beyond that, other nose shapes can be used for special purposes, such as armor-piercing bullets or hollow-point bullets. --Carnildo (talk) 00:54, 30 December 2011 (UTC)
- Bullet itself is also an interesting article on the subject. ←Baseball Bugs What's up, Doc? carrots→ 00:55, 30 December 2011 (UTC)
- In that case, since bullets travel at supersonic speed, wouldn't a sharp, pointed shape allow the bullet to travel fastest through the air and penetrate the deepest into flesh upon impact? Whoop whoop pull up Bitching Betty | Averted crashes 01:06, 30 December 2011 (UTC)
- Many military rifles do indeed fire a pointed bullet called a Spitzer; the name comes from the German for "pointy bullet". The US .30-06 Springfield, British .303 Mark VII, Russian 7.62×54mmR, 7.62×51mm NATO and 5.56×45mm NATO are all Spitzer shaped. Alansplodge (talk) 01:30, 30 December 2011 (UTC)
- In that case, since bullets travel at supersonic speed, wouldn't a sharp, pointed shape allow the bullet to travel fastest through the air and penetrate the deepest into flesh upon impact? Whoop whoop pull up Bitching Betty | Averted crashes 01:06, 30 December 2011 (UTC)
- Ballistics are more complicated than intuitive ideas about how things fly through the air. It's really complicated and you can be assured that very many very smart folks have spent a huge amount of time on it. The hollow-point article linked to earlier is particularly interesting from this point of view: in what seems paradoxical (if you don't understand ballistics), a concave design can cause the most flesh damage, because it expands inside of a target. Penetration of flesh is not that difficult to achieve, in and of itself, if you have a lot of energy behind whatever is flying through the air. Incidentally, if you make the projectile too pointy (e.g. as in a needlegun) it can pass right through the target, which is not as great (from a killing point of view) as expanding inside the target or bouncing around inside the target. --Mr.98 (talk) 01:35, 30 December 2011 (UTC)
- Some bullets travel at supersonic speed. Most handgun bullets and some rifle bullets travel at subsonic speeds. --Carnildo (talk) 02:40, 30 December 2011 (UTC)
- In that case, wouldn't the most efficient bullet design be one that changes shape based on what speed it's travelling at, so that it's pointed when it's travelling at supersonic speed, rounded when it's travelling at subsonic speed, and expanded when it's inside the target? Whoop whoop pull up Bitching Betty | Averted crashes 15:39, 30 December 2011 (UTC)
- That would not be very efficient from a cost point of view. Small arms ammunition is designed to be cheap, simple, lightweight, and reliable, for use by the so-called "line infantry" (... or at least, its modern equivalent). According to the BBC, 14 billion rounds of small arms ammunition are produced each year. Each cartridge, on average, costs much less than one dollar, to make up the $3 billion ammunition market. More advanced armaments exist, employing all sorts of sophisticated technologies; but not for the small arms market, where price and scale and portability are tantamount; individual performance is less important than average performance at scale. Nimur (talk) 16:46, 30 December 2011 (UTC)
- Bullets that expand or disintegrate inside the body were specifically banned by the Hague Convention of 1899. Alansplodge (talk) 18:03, 30 December 2011 (UTC)
- That would not be very efficient from a cost point of view. Small arms ammunition is designed to be cheap, simple, lightweight, and reliable, for use by the so-called "line infantry" (... or at least, its modern equivalent). According to the BBC, 14 billion rounds of small arms ammunition are produced each year. Each cartridge, on average, costs much less than one dollar, to make up the $3 billion ammunition market. More advanced armaments exist, employing all sorts of sophisticated technologies; but not for the small arms market, where price and scale and portability are tantamount; individual performance is less important than average performance at scale. Nimur (talk) 16:46, 30 December 2011 (UTC)
- In that case, wouldn't the most efficient bullet design be one that changes shape based on what speed it's travelling at, so that it's pointed when it's travelling at supersonic speed, rounded when it's travelling at subsonic speed, and expanded when it's inside the target? Whoop whoop pull up Bitching Betty | Averted crashes 15:39, 30 December 2011 (UTC)
- It is an often repeated myth that Expanding bullets are banned per se. Just look at what your friendly local cops carry around.--Aspro (talk) 19:26, 30 December 2011 (UTC)
- They're banned in warfare under the Hague Convention. Neither Hague nor Geneva apply to domestic situations.
- ALR (talk) 10:36, 31 December 2011 (UTC)
- It is an often repeated myth that Expanding bullets are banned per se. Just look at what your friendly local cops carry around.--Aspro (talk) 19:26, 30 December 2011 (UTC)
- That’s what my last post put into context; for the benefit of the OP.--Aspro (talk) 18:12, 31 December 2011 (UTC)
Oddly strong magnetic force
I was installing a new hardrive in my PC the other day when I felt a resistance in my hand as I brought the new drive toward the case. It was at least a foot away at the time so I thought I must have imagined it. Waving the drive through the air several times I descovered a couple of "pockets" of resistance in the air, one of these was at least two feet away from the case, the other hard drives or any other metal object that it could possibly have been interacting with.. are these rare earth magnets realy so strong? I have never expereinced this before, and I've often stacked drives far closer to eachother. e.g. on top of eachother on a shelf and even at such close proximity never felt any such magnetic interaction.. could it be an abnormally strong magnet in this new drive, and could it adversely affect nearby devices? Benjamint 03:25, 30 December 2011 (UTC)
- I doubt it would be the magnet. Was the drive plugged in at the time? I suspect you might have felt the centrifugal force of the spinning platters, that can be strange and feel quite "strong" if you haven't felt it before. I can imagine how you could mistake it for appearing only in "pockets" since you only feel it if you move the drive in a particular way. Vespine (talk) 04:05, 30 December 2011 (UTC)
- I'd recommend a little experiment: Bring the old HD to a place away from other metals. Move a compass around it at some convenient distance and note down the maximum change of the needle, and the distance. Repeat the same with the new HD. If the numbers are significantly different then we have something quantitative to discuss here. — Sebastian 04:16, 30 December 2011 (UTC)
- I've taken a number of old HDDs apart, and from my experience with the magnets, their field (as far as it can be felt by hand when they're brought closer together) extends a few centimetres. A few feet is completely the wrong scale. For a fun look at the topic, see Daniel Rutter's article for quotes like:
--Slashme (talk) 05:55, 30 December 2011 (UTC)You're not going to be lifting any Toyotas with a five buck magnet from anywhere. Nails will hop up only about an inch to hit the strongest of the magnets in the ForceField grab bags. In contrast, ferromagnetic objects of all types will fly across a room to make friends with an MRI machine, as occasional accidents attest.
- centrifugal force sounds plausible, i should have thought of that. Benjamint 08:49, 30 December 2011 (UTC)
- It's actually the Coriolis effect, not a centrifugal force. It is very noticeable with a gyroscope, and is probably detectable from a fast-spinning hard drive. Dbfirs 14:18, 31 December 2011 (UTC)
- You are probably right that it's not technically centrifugal force, but I am not convinced that it is Coriolis either, the examples given in the article do not resemble the gyroscopic effect I'm trying to describe at all. And none of the articles about gyroscopes mention Coriolis. I think most people would understand what is meant by centrifugal, even if it is not the correct term. I actually still can't find the correct term.. Looking for gyroscope and coriolis i found this article which describes the effect in ballistics, calling it gyroscopic spin drift, but even after finding the wiki section it doesn't specify what the causal effect is called. Vespine (talk) 23:11, 2 January 2012 (UTC)
- Both "centrifugal" and "Coriolis" "forces" usually refer to fictitious forces "observed" in a rotating frame of reference, so that wouldn't apply here. Perhaps "gyroscopic effect" would be a better description, but we don't have an article. Dbfirs 00:21, 3 January 2012 (UTC)
- You are probably right that it's not technically centrifugal force, but I am not convinced that it is Coriolis either, the examples given in the article do not resemble the gyroscopic effect I'm trying to describe at all. And none of the articles about gyroscopes mention Coriolis. I think most people would understand what is meant by centrifugal, even if it is not the correct term. I actually still can't find the correct term.. Looking for gyroscope and coriolis i found this article which describes the effect in ballistics, calling it gyroscopic spin drift, but even after finding the wiki section it doesn't specify what the causal effect is called. Vespine (talk) 23:11, 2 January 2012 (UTC)
Maximum sustained G-force a human can endure for long periods?
Suppose a human live in a space station attached to a rocket that accelerate for long periods (weeks) to have to deal with eat, sleep, control tasks etc. What is the maximum acceleration that can be sustained continuously before the human body won't work properly? Like being unable to digest food, breathe, overload heart etc. Electron9 (talk) 08:43, 30 December 2011 (UTC)
- Keep in mind that acceleration is change in speed over time. I have trouble imagining that a space station would be continuously increasing its speed over a period of weeks. As far as how much G-force a human can handle, rocket sled might have some answers. Also g-force. ←Baseball Bugs What's up, Doc? carrots→ 09:53, 30 December 2011 (UTC)
- I wouldn't call such a thing a space station, but it is plausible that an interstellar spacecraft might do something like that. There are existing and planned spacecraft for travelling within the solar system using ion drives that accelerate for long periods, although their acceleration is much less than 1g. You could have a similar system for interstellar travel where you accelerate at more than 1g (although we don't have the technology to do that yet). 2g for a week gets you to 4% the speed of light, which is the kind of speeds you need to achieve to make interstellar travel feasible (examples of reaching higher speeds would require me to take into account special relativity, which I don't feel like doing - I expect you can find online calculators with some simple googling, just be careful about what reference frame the acceleration is measured in). --Tango (talk) 14:32, 30 December 2011 (UTC)
- 4% of the speed of light hardly seems practical for interstellar travel. At that speed it would take over 100 years to get to the nearest star, and chances are a faster ship launched later would pass you on the way (pointing and laughing as they pass). StuRat (talk) 16:30, 30 December 2011 (UTC)
- True, but it's also about the limit of what you can calculate without taking special relativity into account. If you can maintain 2g for a week, you can probably maintain it for a month without too much extra difficulty (your fuel requirements would increase exponentially if you were using a traditional rocket, but you probably couldn't manage a week with that kind of approach anyway), by which time you are getting up to speeds where travelling between stars within a human lifespan is possible. Getting up to speeds where you aren't likely to be overtaken by the next generation of craft is an interesting issue and not one that is easy to figure out. Once you reach large proportions of the speed of light, you are pretty safe, though. --Tango (talk) 20:31, 31 December 2011 (UTC)
- And then there's the issue that nobody wants to fund a project, knowing it will likely be overtaken by faster craft on the way. But, on the other hand, if nobody funds the first project, the knowledge base will not exist to build the faster ships, later. And we can't develop near-light-speed travel for zipping around our solar system, and then apply that the interstellar travel, as the G's would kill us on the short trips. Quite the Catch-22. This somewhat reminds me of the issue of subsonic versus supersonic commercial airplanes. Supersonic seems to make sense for long trips, but such planes are lousy at shorter, subsonic flights, so developing a fleet of SST's is impractical, dooming us all to long subsonic flights. StuRat (talk) 22:11, 31 December 2011 (UTC)
- We have an article on this subject (of course): Space travel using constant acceleration. --Tango (talk) 14:33, 30 December 2011 (UTC)
- That article has kind of a conversational tone that suggests it was lifted from elsewhere. Be that as it may, if I'm understanding correctly, the problem with a high g-force has to do with "pushing" against a gravitational field. But if you're in deep space, you wouldn't have that problem, would you? ←Baseball Bugs What's up, Doc? carrots→ 15:33, 30 December 2011 (UTC)
- To redeploy a famous quote: Bugs, you're not even wrong. {The poster formerly known as 87.81.230.195} 90.197.66.116 (talk) 15:57, 30 December 2011 (UTC)
- I would expect that we can't handle more than 1.1 or 1.2 g for any extended time period. Perhaps with special equipment, like a pressure suit that compresses and relaxes to help pump the blood, we might be able to do slightly more. The work load a person can handle would also be reduced, as just walking around would be exhausting. Instead of sitting (on the relatively small buttocks), people would need to recline on their backs, as astronauts to in launch position, to distribute the force over a wider area. Special shoes and/or floor coverings would also be needed, to reduce the stress on the feet while walking, and to protect them from falling objects. StuRat (talk) 16:27, 30 December 2011 (UTC)
- Do you have any references to support your statements, StuRat, or is that just speculation? (And if speculation, do you have any professional or educational experience or qualifications that might qualify you to offer such guesses?) TenOfAllTrades(talk) 17:27, 30 December 2011 (UTC)
- To paraphrase your previous comment to me, you've wasted my time more than enough already. You're over your quota, and I have no intention of getting into a debate with someone as rude as you. StuRat (talk) 03:18, 31 December 2011 (UTC)
- That's "no", then? TenOfAllTrades(talk) 03:24, 31 December 2011 (UTC)
- It's "no intention of taking the bait", go stalk somebody else. StuRat (talk) 03:33, 31 December 2011 (UTC)
- I have no idea what this is all about, but I'd also like to see corroboration of the 1.1 - 1.2 g figure. A high-g environment is not like obesity in many ways: obese bodies have adapted to their new weight, but obesity is also harmful for reasons other than the increased weight itself. For example, I think cardiovascular problems, higher risk of cancer, and higher risk of diabetes are more due to the poor diet and sedentary lifestyle than due to gravity. The question of what happens to the human body under increased gravity, but without the diet and lifestyle associated with obesity, is an interesting question that I'm not sure any research addresses. --99.237.252.228 (talk) 08:29, 31 December 2011 (UTC)
- I believe that the need to pump more blood through more tissue is a large factor in cardiovascular disease. This requires greater blood pressure variations to distribute the blood, which causes damage. On cancer and diabetes you may well be right, though. A better analogy might be giants, of normal weight for their height. I believe they do suffer health consequences as a result of their size, even with a healthy diet.
- Ten likes to stalk me and post comments like that. If I then posted a list of qualifications, he would dismiss anything less than a Nobel prize in that area as insufficient. You will notice he posted no qualifications while demanding mine. And, he has posted nothing helpful towards answering your questions. So, I ignore him. StuRat (talk) 20:17, 31 December 2011 (UTC)
- My qualifications (or references that I might provide) would be relevant if I were offering a different answer, or asserting that StuRat's response was definitely incorrect. On the other hand, it doesn't take specialized skills to notice that his response contained no references supporting his claimed answer. Irrelevant obfuscation about Nobel laureates notwithstanding, it appears that in addition to not basing his response on any real research that he did, he also lacks any specific medical or aeronautical training that might qualify him to offer a professional expert opinion. In other words, he's mad that someone noticed that he posted a response (probably an incorrect one, based on the replies below from editors who actually did bother to find real sources) that he made up out of whole cloth, and that he got called on it. I have called him on such answers before; he has a habit of offering authoritative-sounding responses that aren't based in any real research or knowledge, and he doesn't seem to realize that his idle speculation at our Reference Desk is harmful. TenOfAllTrades(talk) 21:13, 31 December 2011 (UTC)
- Based on StuRat's response to my query, it appears that the 1.1-1.2g figure is something he made up without doing any research at all; it's probably best to ignore it. TenOfAllTrades(talk) 19:32, 31 December 2011 (UTC)
- StuRat's 1.1 or 1.2 g limit seems overly pessimistic. A patient who is class I obese (BMI 30.0–34.9) weighs up to 1.89 times as much as their "normal weight" (BMI 18.5–24.9). -- 49.229.138.140 (talk) 17:33, 30 December 2011 (UTC)
- So how high can BMI go from normal to obese for a long period without destroying the joints etc? Electron9 (talk) 20:53, 30 December 2011 (UTC)
- I can only note that while osteoarthritis is an issue with the obese, type 2 diabetes mellitus and cardiac problems (from DVI to MI) seem to be more serious, and it is not clear how far the high-g vs. obesity comparison would hold. Most issues with obesity are due to physiological effects well beyond just the weight of the excess tissue, while high-g would introduce a head-to-tow blood pressure variation not present in obesity. (prev. posted as 49.229.138.140 ) -- 110.49.224.36 (talk) 02:44, 31 December 2011 (UTC)
- Note that the bodies of the obese have made various adjustments to carry their weight, as they gained it, such as increased muscle mass. I was assuming no such adjustments were made by the astronauts ahead of time, but perhaps you could have them live in a centrifuge or wear weights to bulk up for such an experience. The obese also suffer health problems and can't do as much activity, so are we saying it's OK to damage the health of the astronauts and have them be less productive ? StuRat (talk) 03:07, 31 December 2011 (UTC)
- A report from The Ohio state university on page 11, figure 6 hints that 4G is the maximum for period of 25 seconds, and the diagram suggest it can be sustained. If digestion, lungs, blood system, judgement etc will work for weeks in 4G is another matter ;) Electron9 (talk) 17:11, 30 December 2011 (UTC)
- They said that if 4G can be maintained for 25 seconds they can conclude from that it's fine indefinitely ? That's quite an extrapolation. StuRat (talk) 21:26, 31 December 2011 (UTC)
- Assuming high-g constant acceleration space travel is possible (given all its technical problems), accelerating at over 1 g does not buy you much in terms of total travel time for interstellar trips, though it does reduce the subjective time for the travelers. If we can trust the relativistic calculations done by this space math calculator, a 20 light-year trip at 1 g (half accelerating, half decellerating) would take 21.87 years (6.05 years subjective time), while at 10 g it would take 20.21 years (1.04 years subjective time). So it would seem that human adaptability to long term high-g environments would be less a factor in determining appropriate transportation than in deciding what constitutes a habitable planet. (I see that article discusses habitability for life in general, not humans specifically.) -- 110.49.224.36 (talk) 03:21, 31 December 2011 (UTC)
- That calculator seems a bit iffy. When I used low enough accels and distances to avoid relativistic effects, the numbers didn't seem to match Newtonian math. StuRat (talk) 03:31, 31 December 2011 (UTC)
- Is it a display issue? If you feed the "Long Relativistic Journeys" (acel/decel) calculator with a = 1 g & d = 980.665 m, it appears to claim that t = 6.342 years, but if you scroll within that result box, you see 6.342000000000084e-7 years, or 20 seconds. (10 seconds accelerating for the first 490.3325 m, 10 seconds decelerating for the second 490.3325 m.) -- 110.49.224.96 (talk) 07:21, 31 December 2011 (UTC)
- Yes, that may be the problem. StuRat (talk) 20:05, 31 December 2011 (UTC)
- 110.49: the calculations look correct, because the spaceship would be travelling at nearly light speed under even 1g acceleration, but how did you arrive at the conclusion that "human adaptability to long term high-g environments is less a factor"? If you're on the spaceship, it would make a big difference if you arrive in 1.04 years or 6.05 years. I, for one, would sign up for the 1-year trip if I can survive it, but would go insane if I was stuck on a ship for 6 years! Also, you'd need 6 times as much food, water, air, games to kill boredom, and the like at 1g than at 10g. For people on Earth or at the destination planet, the difference between 21.87 and 20.21 years is insignificant, so the only consideration is really the subjective time felt by the astronauts. --99.237.252.228 (talk) 08:48, 31 December 2011 (UTC)
- At 10g you won't be playing any games, unless you consider "try to keep from passing out" a game. :-) StuRat (talk) 20:05, 31 December 2011 (UTC)
- You are right; I shouldn't have dismissed the importance of the subjective travel time. In this particular case, however, I'd rather risk six years of potentially going insane than one year of going squish. -- (110.49.224.96 finally logged in) ToE 10:14, 31 December 2011 (UTC)
- Poul Anderson's Tau Zero is the best science fiction example of a one g constant acceleration trip set in a universe without FTL drive. For higher-g, long duration accelerations, the only example which comes to mind is Robert A. Heinlein's juvenile Have Space Suit—Will Travel, in which the narrator is kidnapped by an alien "Wormface" and whisked off to Pluto at 8 g. Heinlein steps us through our hero's calculations in his lonly Plutonian cell, where he determines that the trip took five and a half days at 8 g. He is then quite surprised to realize that it would only have taken fifteen days at 1 g. "It seemed to me that it ought to take at least eight times as long at one gee as at eight — more likely sixty-four." He then realizes why t is scaling inversely to the square root of a in s = 1/2 a t2, "... because the more boost, the shorter the trip, and the shorter the trip the less time in which to use the built-up speed." Back to the OP's question, the protagonist was unconscious for most of the trip due to the effects of the acceleration. In the words of "Fats", one of Wormface's henchmen, "Five days at eight gravities ain't no joy ride." -- ToE 10:14, 31 December 2011 (UTC)
- Also relevant is our article Liquid breathing#Space travel. -- ToE 10:29, 31 December 2011 (UTC)
- Also ECMO with liquid immersion (and cavity filling) with a non-oxygen carrying liquid, overcoming the density issues with Perfluorocarbon fluids. -- ToE 10:44, 31 December 2011 (UTC)
I suspect that by gradually increasing the g-load over a period of months or even a few years, you can let physically fit people adapt to living permanently at a few g's. In case of low oxygen, you can actually adapt to live permanently at levels that will make you pass out within minutes if oxygen levels were lowered immediately to that level. Another example: if you increase your fitness by exercising for longer and at higher intensisty, you will likely reach a point where forced exercise at that level before you started to exercise using e.g. amphetamines to overrule your bodies defenses against over exertion, would have been fatal. So, the daily routine of physically fit people is already well within the death zone of the same persons in an unfit couch potato state (but note that Wikipedia calls this latter state the norm, while calling the fit state a diseased state, see the athletic heart syndrome article). The g-load that will kill you within minites is quite large, the g-force that will make you pass out is typically somehwere between 3 and 8 g's, so you could speculate that such large g-loads that would incapacitate you within minutes or even kill you, could actually be sustainable on the long. Then a much more conservative guess would be that g-loads below 3 g's that typically don't incapacitate you immediately could be survivable on the long run, if you give your body the necessary time to adapt to it. Count Iblis (talk) 01:26, 1 January 2012 (UTC)
- One factor to consider is that it might be quite difficult to sleep at high g's. Your system couldn't "relax", because it needs to keep beating your heart quickly to distribute your blood. And you'd need to constantly shift positions to avoid pressure sores (although a special bed might avoid that or turn you continuously). So, sleep deprivation could become an issue. StuRat (talk) 03:44, 2 January 2012 (UTC)
Would any surgeries or maybe nanotechnology or bioengineering be imaginable to make us more resilient to high g travel? Bastard Soap (talk) 17:28, 2 January 2012 (UTC)
Wouldn't full body immersion solve most of these issues? Greglocock (talk) 21:05, 2 January 2012 (UTC)
What'll it take to upgrade landline networks to allow texting to and from landline phones?
Best Buy keeps telling me that none of their landline phone models for sale have texting capabilities.
I'm afraid that I've vowed never to own my own landline until texting capabilities arrive to them.
What will it take to give landlines the capability to send and receive texts? Thanks. --70.179.174.101 (talk) 09:17, 30 December 2011 (UTC)
- I'm not sure where you live, but that's been available in Australia since at least 2005 - see this article about the national provider Telstra introducing the service. It even talks about it being available on public payphones. I can't say about elsewhere in the world, but I'd be pretty sure this wasn't invented in Aust. --jjron (talk) 11:07, 30 December 2011 (UTC)
- I remember this being offered I believe before 2005 in Malaysia. ([5] suggests it was 2004 although I thought it was before then.) Of course the key point Jjron touched on, it obviously depends on your telco more then your phone. If they don't already have it, I have some doubts it will be added to POTS landlines particularly in a place like the US. Digital ones over cable, DSL, fibre etc is already supported by some providers. I presume some VoIP phones support SMS but I'm not aware and a search doesn't suggest there's any real standard for SMS over Session Initiation Protocol or some other standard way for the VoIP hard phones to receive and send SMS. On the other hand, it's not clear to me why the OP wants SMS over their 'landline' anyway. Nil Einne (talk) 11:42, 30 December 2011 (UTC)
- Which country? For textable landlines in the UK, see here for example.--Shantavira|feed me 12:33, 30 December 2011 (UTC)
- Quickfix, use a modem. The other way is to replace the line card at the telephone exchange with that that can handle this function. Otoh, maybe ISDN can be made to deal with this using it's 9600 bit/s control line. Electron9 (talk) 16:54, 30 December 2011 (UTC)
- A fax machine of sorts has been able to this for over 145 years. If you mean texting in the same sense as a mobile phone would work, BT in the UK has provided that service for their home customers for the last few years. That suggests it is a feature provided by the telephone network that you should be looking for first. Astronaut (talk) 02:19, 31 December 2011 (UTC)
- By the way, the IP geolocates to Auburn in metro Topeka, Kansas. Nyttend (talk) 05:28, 31 December 2011 (UTC)
- Your question seems confusing to me: what do you need? a landline phone with texting or a landline with texting? Lanlines phones with texting seems to be pretty uncommon, but texting through the landline network is hardy new news. 88.8.75.198 (talk) 19:41, 31 December 2011 (UTC)
"Formaldehyde appears to be a useful probe for astrochemists due to its low reactivity in the gas phase and to the fact that the 110←111 and 211←212 K-doublet transitions are rather clear."
What is the 110←111 and 211←212?Curb Chain (talk) 09:57, 30 December 2011 (UTC)
- (ec) They are spectral lines - characteristic colors of light that formaldehyde molecules emit when heated. They are described in this section of the article: 6.2 cm and 2.1 cm electromagnetic waves - in other words, on the low end of the microwave spectrum (or high end of the UHF band). We have an article on radio astronomy to give you some background, and you may also want to read emission spectrum if you're unfamiliar with the basics of chemical spectroscopy. Nimur (talk) 17:08, 30 December 2011 (UTC)
- Follow the link to the article on Interstellar formaldehyde which explains it - and contains further links to explain ground state and rotational transition. Rmhermen (talk) 17:07, 30 December 2011 (UTC)
- Both articles don't mention the notation though. If Interstellar formaldehyde explains this, it doesn't explain the formulae for notation/nomenclature.Curb Chain (talk) 03:12, 31 December 2011 (UTC)
Cross-Coupled Oscillator Driving Induction Heater
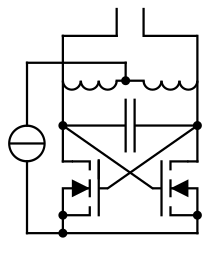
I've been thinking of building an induction heater for a while now, but there's something bothering me.. Most people build an LC tank circuit with the induction coil and some capacitors and drive it with powerful transistors. To tune in to the right frequency a separate circuit is used. Example 1: http://hildstrom.com/projects/inductionfoundry/index.html Example 2: http://www.mindchallenger.com/inductionheater/ The burning question is: why not use a cross-coupled LC oscillator (or similar oscillator) with the coil as the inductive heater coil?! It will naturally resonate at the right frequency and powerful MOSFETs can keep it operating at high powers! Any ideas? Duga3 (talk) 17:58, 30 December 2011 (UTC)
Self tuned invertors for this application are a bad idea - especially if you are inexperienced. A separate frequency determining drive circuit gives you independent and therefore greater control over a multitude of circuit parameters. Also, don't forget that you'll have a sine wave across the tank, but the MOSFET currents for this must be narrow pulses "pumping" each sine peak, or you'll waste most of the power in the MOSFETs and not in the load/workpiece. Pulse width is easily predicted and controlled with a separate oscillator drive, but difficult and probably impossible with simple self oscillating circuits unless the MOSFET gate ratings are exceeded. Looking at it another way, you should be driving the MOSTFET gates with rectangular pulses, but simple cross coupling drives the gates with sine waves - not only does this directly lead to poor efficiency, it also leaves the MOSFETS open to parasitic oscillation at very high and useless frequencies. I have designed self-oscillating invertors using PNP transistors, so they can be made to work, but I would not try it for this application. They do have a theoretical advantage in that a short circuit load or gross overload should stop oscillation - but in practice heavy loads still write off the transistors. — Preceding unsigned comment added by 124.182.5.136 (talk) 10:00, 31 December 2011 (UTC)
- That does makes sense, thanks for your help. Duga3 (talk) 01:19, 5 January 2012 (UTC)
How far away could Seti detect us?
Say there's an alien civilization basically identical to ours, with the same amount of radiowave output. How close would that civilization have to be in order for SETI to pick it up? (I suspect a spectrum of distances, from definitive to... less definitive). Goodbye Galaxy (talk) 19:32, 30 December 2011 (UTC)
- I'm not sure of the answer (not an expert), but you may be interested in Active SETI, which covers this. Meelar (talk) 19:54, 30 December 2011 (UTC)
- And on a semi-related note, here's an article about building a beacon to signal interstellar civilizations. Astronomy Now link. Meelar (talk) 20:04, 30 December 2011 (UTC)
- Depending on your "us", the distance is surprisingly short. IIRC, the Arecibo Observatory could detect Earth's general non-directional radio emissions at a distance of no more than a light-year or two. On the other hand, Arecibo is also a powerful directional radio transmitter (it's been used for radar surveys of the solar system), and could detect itself at a fairly long distance (I've heard 30 light-years and 1000 light-years, but can't find references for either). --Carnildo (talk) 01:18, 31 December 2011 (UTC)
- Update: According to this page, the Arecibo antenna could detect the Arecibo Message "just about anywhere in the galaxy", for a distance of around 100,000 light-years. --Carnildo (talk) 01:24, 31 December 2011 (UTC)

- This question is such a natural one that I am amazed I've never seen it fully addressed; you'd think it would be mentioned in every SETI discussion. The detection range will, of course, be much greater for directed signals, as with Active SETI. Last month, the WP:RD/S question "Radio distance" asked about the effective range of interstellar communication. Based on the 10 TW EIRP of Arecibo Observatory's 2380 MHz signal, this 1981 reception range chart, and subsequent receiver sensitivity improvements suggested by numbers given in the section SETI#MOP and Project Phoenix, I estimated that the Arecibo message could conceivably be detected by an equivalent civilization at its target some 25,000 light years away (assuming they will be listening in just the right distance at just the right time).
- Active SETI aside, military radars can be quite powerful and well focused. NAVSPASUR's Lake Kickapoo master transmitter puts out 768 kW of 216.983 MHz CW, with an EIRP of 6.3 GW suggested on some discussion boards (couldn't find an RS). (Any complete discussion should also mention the detection range of our isotropic transmissions (including the 1936 Berlin Olympics transmission mentioned in Contact.) -- ToE 02:03, 31 December 2011 (UTC)
- On practical maximum distance is N light-years, where N is the number of years for which we have been transmitting radio waves - currently about 125. Any further away than that our radio signals haven't reached yet, so we can't be detected, no matter how sensitive/powerful the receiver. Obviously this maximum distance increases at the rate of 1 light-year per year. Mitch Ames (talk) 02:22, 31 December 2011 (UTC)
- Yes, but I don't think the OP is actually interested in detecting humanity, but in detecting other humanity-like civilisations. If such a civilisation is 500 ly away and reached our level of technology 500 years ago, then whether or not we can detect them will depend on whether or not we could detect us at a distance of 500 ly. --Tango (talk) 20:24, 31 December 2011 (UTC)
- say that this civilisation was 30 years ahead or behind us technologically wise-that could change everything. the chances of it being exactly at the same point technologically is tiny. — Preceding unsigned comment added by 109.152.106.203 (talk • contribs) 20:58, 2 January 2012 (UTC)
Beyond the speed of light
Could there be something like "speed of space", since basically the universe is space in constant expansion and photons can't be present at distances greater than 13.7 billion light years at any given point? Gravitoweak (talk) 23:43, 30 December 2011 (UTC)
- I'm not sure I follow you, but the article Hubble's law may have some reading which may interest you. --Jayron32 23:52, 30 December 2011 (UTC)
- We do have the articles metric expansion of space and cosmic inflation. Are you asking what units this expansion is measured in? -- 110.49.224.36 (talk) 03:30, 31 December 2011 (UTC)
December 31
How -or why- do interleukins produce fever?
How? — Preceding unsigned comment added by 88.8.75.198 (talk) 02:14, 31 December 2011 (UTC)
- Fever#Pathophysiology seems to cover some related issues. --Jayron32 02:21, 31 December 2011 (UTC)
Shaving with boiling water
Most of the time I use an electric razor to shave, but I was having a wet shave this morning and got to thinking that my father used to always use boiling water to dip his razor in when having a wet shave. For the record he used a safety razor, not a cut-throat (and, if it matters, we are just talking about shaving the beard area). I haven't noticed this done elsewhere, and just checking our shaving page, as well as some 'how to have a good shave' sites on the internet, I can't see it recommended to use boiling water. I can guess that there may be some theory about the heat helping to swell the whiskers or improve the efficiency of the cut or something, but does anyone know any more about this, or whether there's any possible scientific validity to his old technique? --jjron (talk) 03:45, 31 December 2011 (UTC)
- Possibly to kill bacteria that might be on the blade? ←Baseball Bugs What's up, Doc? carrots→ 03:55, 31 December 2011 (UTC)
- That would be my guess, although it seems unnecessary. I've cut myself shaving many times, and never had it get infected. The risk of burning yourself would seem to outweigh any benefit. StuRat (talk) 03:58, 31 December 2011 (UTC)
- Hah, just reminded me of a classic Australian poem from 1892 about just that: The Man from Ironbark (full text here). Wonder if it could have come out of there, or if there's some historical precedent to that. --jjron (talk) 05:13, 31 December 2011 (UTC)
- Shaving is more dangerous than you think, StuRat. While the purpose of the antiseptic agent in aftershaves is to prevent infection of cuts, deaths from infected shaving cuts are not unknown. Notable victims include Russian composer Alexander Nikolayevich Scriabin (it might have been a boil), Henry David Thoreau's brother John, Egyptologist George Herbert, 5th Earl of Carnarvon (yes, the Curse of Tutankhamun is death by shaving!), and U.S. Representative Michael F. Farley (via anthrax from a shaving brush). While we are all glad that you have survived so far, StuRat, your complacency is dangerous. Personally, I follow the lead of the men from Ironbark (where "flowing beards are all the go") just to be safe. (I've always heard that Reserve Constable Albert Alexander, the first patient treated with with injections of penicillin (he wasn't treated until he was already severely ill, and while he responded well to the drug, he died shortly after the supply ran out), was suffering from an infected shaving cut, but our article claims that he was accidentally scratched by a rose thorn in his mouth. I suppose that now I'll have to stop eating roses.) -- ToE 05:43, 31 December 2011 (UTC)
- Actually that's a good point (and I should have thought of it earlier). Although it was before I knew him, I have a friend who spent months in intensive care close to death and ultimately lost close to a year of his life after a shaving nick at the hairdresser (shaving the back of his neck) went bad. It wasn't that long ago either, I think it was probably sometime in the late '80s. --jjron (talk) 06:40, 31 December 2011 (UTC)
- I can't say with scientific accuracy, but I use a cutthroat and dipping it in boiling water between strokes feels more comfortable. @Baseball Bugs, I almost invariably get a rash if I don't use aftershave( I use spirit). @StuRat, The blade and the layer of water actually cools by the time it reaches my chin and feels "just warm".
- I think the hot water may make the cream more effective and unclog the edge of the blade. A little scum at the cutting edge seems to make a big difference in the ease of shaving. Though I cannot discount the chance that I just don't like cold steel against my neck. Staticd (talk) 05:31, 31 December 2011 (UTC)
- I personally dip the razor in hot water (not usually boiling, but fairly hot) because the hot blade contrasting with the coolness of my menthol-containing shaving cream works wonders for waking me up in the morning. I have never considered other possible advantages to this practice - it just feels so much better to me. Equisetum (talk | contributions) 11:33, 31 December 2011 (UTC)
- For some reason I don't really have that much facial hair, and only need to shave once every two days to feel comfortable, and can use a single-use disposable razor for three months. And I usually run it under hot water before using it, it feels much better and the skin response is much better than when using cold water (i. e. shaving during camping or on a gas station in the middle of nowhere, if I have to, can be a drag). I saw my father dipping his razor in boiling water before shaving, and he did it exactly because of the germs. --Ouro (blah blah) 17:18, 31 December 2011 (UTC)
- He actually boiled a kettle before shaving? --Tango (talk) 23:26, 31 December 2011 (UTC)
- He got some from the kitchen usually, you know the morning routine, coffee, a cigarette and shaving. --Ouro (blah blah) 20:09, 1 January 2012 (UTC)
- He actually boiled a kettle before shaving? --Tango (talk) 23:26, 31 December 2011 (UTC)
- For some reason I don't really have that much facial hair, and only need to shave once every two days to feel comfortable, and can use a single-use disposable razor for three months. And I usually run it under hot water before using it, it feels much better and the skin response is much better than when using cold water (i. e. shaving during camping or on a gas station in the middle of nowhere, if I have to, can be a drag). I saw my father dipping his razor in boiling water before shaving, and he did it exactly because of the germs. --Ouro (blah blah) 17:18, 31 December 2011 (UTC)
- Us girls know that it is better to shave in hot water as it causes the hairs to extend ever so slightly more. Consequently, when the shaved area cools, the hair retracts just below the skin line, and the area stays smoother longer. Mind you, we all shave in the shower anyway. Elen of the Roads (talk) 02:09, 1 January 2012 (UTC)
- Men know that too. If you're having a wet shave, then you make the beard area wet with hot water (unless you don't have any hot water or you think it's macho to use cold water). The hot water, together with the soap/foam, softens the hairs and makes them easier to cut close (I think the softness is more important than making them extend). You wouldn't use boiling water, though, since that would burn your face! This discussion is about dipping the razor in boiling water. I sometimes shave in the shower. It makes it much easier to get a close shave, but there is always the issue of the mirror steaming up. That may not be an issue for you, but shaving your face without a mirror is a little tricky! (Not impossible, though, if you're careful.) --Tango (talk) 04:09, 1 January 2012 (UTC)
- I completely ruined my sideburns last time I tried mirrorless shaving - after that I go with a "designer stubble" look if there's no mirror to hand. More to the point, I have seen suction-cup equipped mirrors designed for shaving in the shower that have a surprisingly effective anti-fog coating, circumventing that problem. Equisetum (talk | contributions) 11:32, 1 January 2012 (UTC)
Prescription first aid kit
I'm blanking this question and all the responses. Serious, people, what are you thinking? We do not give advice on prescription drugs. What drugs to prescribe is a decision for the doctor writing the prescription and we should not be involved in any way. The OP is either a pharmacist or is working for a pharmacist, so should know far more about this stuff than we do anyway (and should know better than to ask random people on the internet this kind of question). Please do not replace this question or any of the responses without a clear consensus on the talk page. --Tango (talk) 14:48, 31 December 2011 (UTC)
- This is being discussed on the Talk Page. Buddy431 (talk) 18:17, 31 December 2011 (UTC)
What fraction of calories in food are typically needed for digestion, e.g. to produce digestive enzymes ?
I found several answers on google, but none of those look very trustworthy
http://wiki.answers.com/Q/How_many_calories_are_burned_during_digestion says "up to 200 calories per meal" Another site (which is apparently blocked here) claims it is always below 100%
I'm looking for a trustworthy, scientific source here (wikipedia will do) 83.134.159.252 (talk) 14:52, 31 December 2011 (UTC)
- Thermic effect of food would be the place to find that sort of info, but that only says that around 10% of food's calories are used to digest it. I can't find any specific figures, presumably due to the difficulty of measuring it. Assuming you don't have journal access, this or this should tell you more (the full versions are available for free). SmartSE (talk) 15:13, 31 December 2011 (UTC)
- I can't see how it can always be less than 100%. There are things you can eat which fool your body into thinking they are food, like artificial sweeteners, but which contain zero or maybe one calorie. In such a case it must use more energy than that to digest. However, if you think this is a good weight loss strategy, think again. Your body will become very hungry for real food after being tricked like this. StuRat (talk) 16:09, 31 December 2011 (UTC)
- Thanks. I was just looking for a good source to use in a discussion, I am not planning to base a weight loss strategy on this. 83.134.159.252 (talk) 06:39, 1 January 2012 (UTC)
Lower resting O2 saturation in endurance athletes
Should endurance athletes typically have lower resting blood O2 saturation than normal (as measured by a pulse oximeter)? Say around 92%. Sławomir Biały (talk) 15:06, 31 December 2011 (UTC)
- I don't see why. They should be able to get the normal saturation with fewer breaths, though. StuRat (talk) 16:11, 31 December 2011 (UTC)
- They only reason I can see for an athlete having low resting sats is if they are doing altitude training, in which case they'll have the same low sats as anyone at high altitude (at least until they are acclimatised properly). If you have been measuring your own sats are are concerned about them, you should see a doctor. They will be able to tell you pretty quickly whether you have anything to worry about. --Tango (talk) 16:33, 31 December 2011 (UTC)
Are lotteries time travel proof?
Let's say you have the winning numbers of a lottery and you travel back in time to purchase a ticket. Doesn't chaos theory mean that your actions in purchasing that ticket would affect the circumstances of ball/number selection selection? That you would throw things askew just enough to reduce your odds of winning? I mean, go into a store and buy a ticket, but in so doing cause every subsequent person to purchase theirs roughly 30 seconds later than they would have thus causing thousands of numbers to be different than they were. Or is your contribution to the events that determine the numbers so small that you'll probably still win? Some lotteries use machine generated numbers. Maybe they could add variables to their number generation like amount of tickets sold and at precise times to better protect against time travellers. Lotteries should be enacting measures to protect against time traveler schemes, I would think. It would be really unfair to the rest of us standard timeline blokes who buy tickets if they didn't. — Preceding unsigned comment added by 90.9.15.204 (talk) 21:38, 31 December 2011 (UTC)
- I'm no expert, but it would seem to me that the chance of event A (purchasing a ticket) affecting event B (lottery drawing) would be dependent on the initial proximity of the events and the elapsed time between the two events, as the effect of event A expands over time. This is related of the Chaos theory#topological mixing aspects of Chaos theory. For example, if you bought your ticket at a convenience store that is later visited by the lottery operator(s), you could easily introduce a change in timing or behavior that causes the operator to run the machine slightly differently. However, if you bought your ticket in a different city shortly before the drawing, there would be a much smaller chance of affecting the outcome. -- Tom N (tcncv) talk/contrib 23:20, 31 December 2011 (UTC)
- You are assuming that time travel actually changes the past. The simplest theories of time travel have the past staying the same. Say you watched the lottery at 6pm and then went back to noon to buy your ticket. You experience that noon twice. Those theories say that the first time you experienced that noon there was a 2nd copy of you buying the ticket. When you go back in time, you are just that 2nd copy that was there all along, so nothing changes. If that's the case, then your lottery scam should work perfectly. --Tango (talk) 23:34, 31 December 2011 (UTC)
- I don't know what you mean by theories of time travel. Time travel is not possible. There is no point in discussing what would happen if you do it. It's like discussing what would happen if two objects could occupy the same space or if we could travel faster than the speed of light. It doesn't make sense. 88.8.76.174 (talk) 23:58, 31 December 2011 (UTC)
- That's a bold assertion, one that a good many scientists appear to have wasted their time attempting to learn otherwise. Our own articles aren't so certain, although not so much as to suggest being able to go back and buy a lottery ticket. Mingmingla (talk) 00:19, 1 January 2012 (UTC)
- Time travel would require some kind of exotic matter or similar that we have no reason to believe exists, but it isn't impossible that it exists. --Tango (talk) 00:59, 1 January 2012 (UTC)
- I don't know what you mean by theories of time travel. Time travel is not possible. There is no point in discussing what would happen if you do it. It's like discussing what would happen if two objects could occupy the same space or if we could travel faster than the speed of light. It doesn't make sense. 88.8.76.174 (talk) 23:58, 31 December 2011 (UTC)
- If I read the winning number for the lottery, then dash down to my basement, select suitable Tesla coils, pieces of loadstone, vacuum tubes, and stone chips from Stonehenge, build a time machine, and travel back to a time before the drawing to purchase a ticket with the winning numbers, the simplest explanation of what would happen next is that a parallel time-space continuum would then follow, in which I had the winning ticket. "Changing history?" No, creating an alternative history. There are "multiverse" theories in which, is you drop the toast, it lands jelly side up in one continuum and jelly side down in a different one, or in which a different continuum exists with a branching at every possible quantum event. Thus there is no contradiction, and no mysterious forces need manifest themselves to "prevent us from changing history." Edison (talk) 00:28, 1 January 2012 (UTC)
- I think the immutable past theory is simpler than the the parallel universe theory, for the simple reason that it doesn't require additional universes. With an immutable past, you can do whatever you like in the past but it won't change anything because the future you did exactly the same things the first time round. There are no mysterious forces involved, it's just a closed loop. --Tango (talk) 00:59, 1 January 2012 (UTC)
- If people from the future could mess with our timeline, their would be a lot more to worry about than who wins the lottery. StuRat (talk) 00:31, 1 January 2012 (UTC)
- If people from the future could travel in time, why aren't they appearing here where (or when?) I am, every now and then? 88.8.76.174 (talk) 03:15, 1 January 2012 (UTC)
- One way to find out time travelers from the feature would be to spot anomalies. Like in the movie "12 Monkeys" where the main character is found on a photograph from World War 1, despite living in present time, especially beating any system with "impossible" odds like the stock market etc.. Maybe the present is just the most probable time point. Electron9 (talk) 06:21, 2 January 2012 (UTC)
"Maybe the present is just the most probable time point." - I like that. Not entirely sure what you meant, but for me, it suggests time as a probabilistic phenomenon: we occupy the present as an electron occupies the most likely position in its cloud - or something... Time as a bell curve, with the present at the peak probability - you have to use a improbability drive to get into the future or past... Sorry, just thinking aloud here Adambrowne666 (talk) 06:37, 2 January 2012 (UTC)
- However, some people are bound to do amazingly well in the stock market just as some people are destined to win the lottery, not because they knew the results ahead of time, but just because so many people attempt it. So how would you distinguish the lucky from the time travelers ? Personally, I'd look for the tin foil clothing :-). StuRat (talk) 06:31, 2 January 2012 (UTC)
- Its depend on the forth you act , and the justis that organis that on the top - the good for last , and money only for good thing anles you act strongly on time travel .Thanks Water Nosfim — Preceding unsigned comment added by 212.199.175.104 (talk) 12:30, 1 January 2012 (UTC)
- I'm sorry, but that doesn't make any sense... --Tango (talk) 13:01, 1 January 2012 (UTC)
- sometims when you travel you prefer to act at cases like life and death and live it on top , staf like money stay on the side and gets like glass -you can look throw them if you not act strong enafh . thanks Water — Preceding unsigned comment added by 77.127.6.242 (talk) 13:27, 1 January 2012 (UTC)
- Water Nosfim appears to be editing from a parallel universe in which the English language developed in a very different way. Dbfirs 17:52, 1 January 2012 (UTC)
- sometims when you travel you prefer to act at cases like life and death and live it on top , staf like money stay on the side and gets like glass -you can look throw them if you not act strong enafh . thanks Water — Preceding unsigned comment added by 77.127.6.242 (talk) 13:27, 1 January 2012 (UTC)
- You wouldn't need to buy the ticket in a store and delay the next customers by so much: instead you can buy a lottery ticket over the internet or mobile phone from a prepaid card. – b_jonas 12:36, 2 January 2012 (UTC)
January 1
happy new year
Habitable exoplanets
How many exoplanets are rocky and in their star's habitable zone? Which ones are the most likely to have life? --108.225.115.211 (talk) 00:15, 1 January 2012 (UTC)
- There are no definite detections of rocky planets in the habitable zone. The closest is Kepler-22b that was announced a few weeks ago. It's in the habitable zone and isn't too much bigger than Earth, so it's possible it is rocky, or at least is covered in a liquid water ocean (that could possibly support life - we don't have anywhere near enough information yet). --Tango (talk) 00:24, 1 January 2012 (UTC)
- Pass! However, the Drake equation gives some sums on the subject in general.--Aspro (talk) 00:24, 1 January 2012 (UTC)
- You have to make wild guesses about what numbers to put into the equation, though, so it isn't much use for anything other than structuring the conversation (which is all Drake invented it for). --Tango (talk) 01:00, 1 January 2012 (UTC)
- Since no one recommended it for reading, try Habitable zone on for size. --Jayron32 04:44, 2 January 2012 (UTC)
- You have to make wild guesses about what numbers to put into the equation, though, so it isn't much use for anything other than structuring the conversation (which is all Drake invented it for). --Tango (talk) 01:00, 1 January 2012 (UTC)
Interrupter gears
Why can't an interrupter gear be used to synchronise an open bolt machine gun? Whoop whoop pull up Bitching Betty | Averted crashes 03:35, 1 January 2012 (UTC)
- Who says it can't? ←Baseball Bugs What's up, Doc? carrots→ 05:13, 1 January 2012 (UTC)
- The article states that they can't, and I was wondering why. Whoop whoop pull up Bitching Betty | Averted crashes 06:05, 1 January 2012 (UTC)
- The article appears to be incorrect. It has no citation for that information. Technically, the official policy on Wikipedia is to remove unsourced material from articles. You may want to discuss this further on the talk-page for the relevant articles. We have several books and external sources listed in the references section, if you want to research further. Nimur (talk) 07:57, 1 January 2012 (UTC)
- Nimur, are you saying that you have heard of interrupter gear based synchronised open bolt machine guns, or are you simply saying that the article is incorrect in that it is not sourced? With a longer time to first fire after being triggered, it seems reasonable that open bolt guns would be harder to synchronize than closed bolt ones, and our article Parabellum MG14 says, "The MG14 was tried with the pioneering Fokker Stangensteuerung synchronizer on the Fokker E.I pre-production prototypes, but the gun's reliability in this installation eventually proved to be unsatisfactory, even though its closed bolt firing cycle was a desirable feature for synchronisation." -- ToE 12:57, 1 January 2012 (UTC)
- The article appears to be incorrect. It has no citation for that information. Technically, the official policy on Wikipedia is to remove unsourced material from articles. You may want to discuss this further on the talk-page for the relevant articles. We have several books and external sources listed in the references section, if you want to research further. Nimur (talk) 07:57, 1 January 2012 (UTC)
- The article states that they can't, and I was wondering why. Whoop whoop pull up Bitching Betty | Averted crashes 06:05, 1 January 2012 (UTC)
- Wwpu, I know nothing of this field beyond what I have just read in the Wikipedia articles (so experts, please speak up), but here is why I would expect an open bolt gun to be very difficult to synchronize. First, note that the firing speed of these guns are typically much slower than the rotation speed of the propeller -- say 700 rounds/minute for the MG14 mentioned above vs. 2,500 to 3,000 rpm for a propeller (see propeller speed reduction unit). For a two bladed prop, this gives 5,000 to 6,000 firing windows per minute. That is one window every 10 to 12 milliseconds, compared with the 86 ms cycling time of the gun. Next, note that the action of the synchronizer gear attempts to trigger the gun at every firing window, and will do so as long as the pilot is holding down the trigger and the gun has cycled to a firing configuration. Finally, consider the different actions. Firing from a closed bolt involves the firing pin striking the primer. The round fires quickly, then the bolt cycles during the remainder of the 86 ms. Firing from an open bolt involves releasing the bolt and having it feed a round into the chamber as it closes and then firing it, a process which presumable takes at least half of the 86 ms cycle. From the time that the open bolt gun has been triggered by the synchronizer to when the round has fired, a propeller blade will have passed in front of the gun four or five times. Add in a variable speed propeller, and you can see how hard it would be to synchronize an open bolt gun. -- ToE 14:00, 1 January 2012 (UTC)
- Our Closed bolt article agrees with your second point ToE; "since the bullet firing from the gun started the firing cycle, it was much easier to set the synchronizer to only trigger the gun when the propeller's blade was not in front of the gun." Alansplodge (talk) 15:34, 1 January 2012 (UTC)
- Following a link in the paragraph you quoted from, I found Lewis gun#Aircraft use: "The open bolt firing cycle of the Lewis prevented it from being synchronized to fire directly forward through the propeller arc of a single engined-fighter". -- ToE 23:42, 1 January 2012 (UTC)
- Our Closed bolt article agrees with your second point ToE; "since the bullet firing from the gun started the firing cycle, it was much easier to set the synchronizer to only trigger the gun when the propeller's blade was not in front of the gun." Alansplodge (talk) 15:34, 1 January 2012 (UTC)
Watts and calories
A question regarding equivalency of units has arisen here. Would someone please take a look? Rivertorch (talk) 06:49, 1 January 2012 (UTC)
- Responded at talkpage post, but would welcome someone else checking figures. --jjron (talk) 08:26, 1 January 2012 (UTC)
- Responded also; the units used were wrong. IRWolfie- (talk) 15:36, 1 January 2012 (UTC)
Given that glass is transparent to infrared, how usefull are thermal (infrared) images of houses in estimating heat loss through windows?
Under everyday circumstances, heat loss from houses is probably dominated by conduction or diffusion and not by infrared radiation emitted by the house. It seems that silica-based glass is transparent to near-infrared ( http://answers.yahoo.com/question/index?qid=20061029200658AAWl7IU ). In that case, a picture taken in near-infrared will merely register the temperature of the room behind the window, and improving the window (Insulated glazing) will reduce conduction or diffusion losses but on the near-infrared picture you will still see the temperature of the room behind the window. 83.134.159.252 (talk) 07:00, 1 January 2012 (UTC)
- Near infrared is not the same as "thermal" infrared. Infrared is an entire range of invisible colors of light - ranging from near infrared (which optically behaves very similarly to regular red light) all the way deep into the terahertz band, where infrared light behaves more like something we'd expect out of a radio-wave. Somewhere in the middle of that spectrum is "thermal" infrared - light that corresponds to the peak emission of the blackbody radiation curve for objects at useful temperatures.
- Usually, the range of wavelengths that are useful for measuring normal household objects are what we call "long wavelength" - around 10µm - infrared. Glass is not very transparent to these wavelengths, which is why greenhouses work. Visible light (and near infrared) can travel through, and the energy is absorbed by the interior; but when thermalized and re-radiated, that energy is now at a wavelength that will not go out the window. This one-way trip for the energy is called the greenhouse effect.
- If you're using an infrared imager that is capturing near infrared (which is easy and cheap to acquire!) ... then, you aren't actually measuring the temperature. At best, your imager may estimate the temperature based on a complicated mathematical model; but it's not going to be accurate. True thermal infrared imagers are much more expensive and difficult to come by. Use caution! There are lots of infrared imagers that aren't useful for measuring temperature. They're still useful for other scientific and aesthetic purposes, just not as a thermometer. Nimur (talk) 09:26, 1 January 2012 (UTC)
- To add to that: when near infrared is used for night vision, you need to have an artificial source of near infrared light. That's why there is often a bright circle in the middle of a night vision video - the light is attached to the camera and that's where it is shining. --Tango (talk) 13:07, 1 January 2012 (UTC)
- Unhelpful hogwash, see Night vision device for accurate information on Tango's tangent. Bred Ivy (talk) 14:51, 1 January 2012 (UTC)
- What I said is accurate. If you look at Night_vision#Night_vision_technologies you will see there are three types. I was describing how active night vision works, which is the type that most uses near infrared. Your comment on my talk page is about image intensification, which don't just use infrared. They also intensify visible light. So "a picture taken in near-infrared", as the OP is asking about, would generally refer to active night vision, not image intensification. --Tango (talk) 15:20, 1 January 2012 (UTC)
- Unhelpful hogwash, see Night vision device for accurate information on Tango's tangent. Bred Ivy (talk) 14:51, 1 January 2012 (UTC)
- To add to that: when near infrared is used for night vision, you need to have an artificial source of near infrared light. That's why there is often a bright circle in the middle of a night vision video - the light is attached to the camera and that's where it is shining. --Tango (talk) 13:07, 1 January 2012 (UTC)
science assignment
please help me to write an introductory part of an assignment in which the table content are 1.male reproductive system 2.female reproductive system 3.fertility 4.menstruation 5.pregnancy 6.parturition 7.lactation. 8.family planning .Rikisupriyo (talk) 08:14, 1 January 2012 (UTC)
- I'm sorry but it is our policy here not to do people's homework for them, but merely to aid them in doing it themselves. Letting someone else do your homework does not help you learn how to solve such problems. That said, our article on Human reproduction might give you an idea for the assignment introduction. Please do not copy it though.-- Obsidi♠n Soul 08:21, 1 January 2012 (UTC)
- Well, we can't do their homework, but we could suggest some ideas for them to explore. StuRat (talk) 01:54, 2 January 2012 (UTC)
- You might want to describe how human reproduction fits into the animal kingdom. The first two chapters apply, to some degree, to any animal with sexual reproduction. Lactation is specific to mammals. Menstruation is limited to certain mammals. Family planning is limited to humans, unless you include animals which only breed when they are eating well. StuRat (talk) 01:54, 2 January 2012 (UTC)
OTC Medication Safety
Are there any well-defined levels of safety that must be demonstrated before a medication can be approved for distribution over-the-counter? For example, I might imagine there could be conventions such as: "The LD50 concentration must be at least 100 times the typical therapeutic dose." Do any such broad standards for safety exist, or do the review panels simply decide the question of safety on an ad hoc basis every time they make an OTC determination? Dragons flight (talk) 10:15, 1 January 2012 (UTC)
- The rules and regulations (not to mention the specific drugs actually sold OTC) can vary quite a bit from country to country. In the United States, OTC drugs are regulated by the FDA, and decisions on when and how OTC drugs may be sold ultimately flow from the recommendations of the Nonprescription Drugs Advisory Committee. The day-to-day work comes from the Division of Nonprescription Clinical Evaluation (DNCE) and the Division of Nonprescription Regulation Development (DNRD) under the Office of Drug Evaluation IV (ODE IV); the roles of each division are pretty much what their names imply—one deals with clinical testing, and one deals with managing the regulations.
- There's an astounding body of legislation and regulations associated with prescription and nonprescription drugs, but approval of drugs for OTC use generally comes down to meeting the following criteria (copied from [6]):
- their benefits outweigh their risks
- the potential for misuse and abuse is low
- consumer can use them for self-diagnosed conditions
- they can be adequately labeled
- health practitioners are not needed for the safe and effective use of the product
- All of those tend to have some wiggle room in them, and require expert judgement about where to draw the line in any given case. The FDA can require clinical testing of OTC candidates to specifically confirm that patients are able to comprehend labels and to self-manage their use of any drug. While other countries will have different rules, I suspect that you'll find that they come down to roughly the same criteria. TenOfAllTrades(talk) 16:34, 1 January 2012 (UTC)
- Medscape has a little about this too. [7]--Aspro (talk) 16:39, 1 January 2012 (UTC)
It also depend if examiners will possible have a future career option at the medicine inventor, who pays research grants, monetary incentives, who select which protocols from clinical trials will be published etc.. A lot of non-obvious lobbying goes on. Electron9 (talk) 06:42, 2 January 2012 (UTC)
- Can you provide more information about this 'non-obvious lobbyng'? I don't see any reason to doubt the integrity of the pharma industry, althou other people are of different opinion. — Preceding unsigned comment added by 88.8.76.174 (talk) 22:11, 2 January 2012 (UTC)
Kepler-22b
Is there any work ongoing to find out more about Kepler-22b? Kepler's observations left a lot of unanswered questions about mass, composition, most of the orbital parameters, etc. and presumably other space- and ground-based observatories will need to do follow-up studies to answer those questions. I haven't been able to find any information about such follow-up studies, though. --Tango (talk) 17:05, 1 January 2012 (UTC)
- You mean Kepler-22b right? Whoop whoop pull up Bitching Betty | Averted crashes 17:27, 1 January 2012 (UTC)
- Indeed! Fixed now - thanks. I got it right in the header, at least! --Tango (talk) 18:45, 1 January 2012 (UTC)
- Some of those parameters are pretty difficult to find out. We have a detailed article Methods of detecting extrasolar planets which gives some info on which methods give what data. The Kepler (spacecraft) detects planets by the Transit method, which only determines a planet's radius. To find its mass, some sort of Radial velocity method must be employed. Unfortunately, this method works best for larger planets. The radial velocity numbers (measured by the Keck 1 observatory on the ground) give an upper bound on the mass, but no lower bound [8]. My understanding at present is that we lack any instruments capable of giving a reasonable radial velocity of a star for a planet this small (and thus the mass is somewhat unclear). I don't really think we have any good way of measuring composition for any exoplanet. We can guess for some planets if we know their density (i.e. we know they're gas giants), but that's about it. For a few very large planets, we can do spectroscopic measurements directly on the planet. HD 209458 b, for example, was determined to have water vapor and carbon monoxide present with spectroscopic measurements. These are not possible on Kepler-22b, and probably won't be for some time. Buddy431 (talk) 23:25, 1 January 2012 (UTC)
Attractiveness by percentile
Assuming that rankers can put people in the ranked group into a roughly linear order, the "total attractiveness" of a person is the function which given a percentile gives the fraction of rankers which found the person to be in at least that percentile (what's this called in statistics?). Can you link me to someplace which shows some real life "total attractiveness functions" (a term I made up)? --193.64.22.148 (talk) 18:26, 1 January 2012 (UTC)
- Maybe I am misunderstanding you, but this hardly seems to be a question about science. There is no science of attractiveness, it is purely subjective and cannot be measured scientifically. Beeblebrox (talk) 20:13, 1 January 2012 (UTC)
- "Cannot" is a strong word... the whole of the social sciences are a best-effort approach to apply the scientific method to subjective topics. In fact, even hard-science journals like Nature have published studies about human perceptions of attractiveness. Here's one example article I found using Google Scholar: Effects of sexual dimorphism on facial attractiveness (Nature, 1998). There are some promising-looking articles from journals I haven't ever read, too: An Objective System for Measuring Facial Attractiveness, Facial attractiveness, developmental stability, and fluctuating asymmetry, and so on. We have an article on the topic of physical attractiveness that can probably point you to more survey articles and published research. Our article lists almost 200 reference works. Nimur (talk) 20:40, 1 January 2012 (UTC)
- Maybe I am misunderstanding you, but isn't the fraction the same as the percentile? If it is, then the function is the identity function, but perhaps you are describing something more subtle. (I'm not a statistician.) Dbfirs 20:47, 1 January 2012 (UTC)
- Maybe I'm miscommunicating, but I was thinking about a situation like "f(more attractive than %x of the population being ranked) = true in the case of %y of rankers". So f(%0) = %100 and f(%100) = the percentage of rankers who consider the person most attractive of all. --~~ — Preceding unsigned comment added by 193.64.22.148 (talk) 21:12, 1 January 2012 (UTC)
- So you want to know something about how different people's perceptions of attractiveness are correlated? That's an interesting question. It's a slightly difficult one to answer, though, because people will often say they think someone is attractive when actually they mean they think they match what one is "supposed" to find attractive. If people are answering like that, then obviously there will be a strong correlation. If people are giving their own genuine opinions, then it might be a lot weaker. I haven't heard of any studies into that, but it wouldn't surprise me if there were some (such studies tend to get mentioned in the press, which means there is probably a lot of funding available for them!). --Tango (talk) 02:15, 2 January 2012 (UTC)
- Maybe I'm miscommunicating, but I was thinking about a situation like "f(more attractive than %x of the population being ranked) = true in the case of %y of rankers". So f(%0) = %100 and f(%100) = the percentage of rankers who consider the person most attractive of all. --~~ — Preceding unsigned comment added by 193.64.22.148 (talk) 21:12, 1 January 2012 (UTC)
- See panel 3 of xkcd comic strip 451: Impostor. – b_jonas 12:26, 2 January 2012 (UTC)
Velocities
Consider a particle moving along the line x = 1. Assume that after t seconds, the particle is at the point (1,t) in the xy-plane. The linear velocity of the particle is clearly (0,1) and its speed 1 m/s. Consider the chord joining (0,0) to the particle at (1,t); this chord makes an angle, measured in radians, with the x-axis of
Does it make sense to consider the angular velocity of this particle, with respect to the centre of rotation (0,0), or do particles have to follow circular arcs to have well define angular velocity? Assuming it does make sense, we see that the angular velocity, measured in radians per second, of the particle is
I understand the meaning of angular velocity for a body rotating about a point or an axis, but in the case of a particle moving along a straight line, what does my ω(t) mean? Is it some sort of "infinitesimal angular velocity"? Also, what do we call the sum of the linear and angular velocities? (It's interesting that the trajectory of the particle is only ever tangent to a circular arc centred at (0,0) when t = 0 — it's tangent to the unit circle at (1,0). In this case the angular velocity ω(1) = 1 matches the linear velocity.) — Fly by Night (talk) 23:19, 1 January 2012 (UTC)
- The only significance I can see to calculating the angular velocity, momentum, etc., under such conditions are if the particle may, at some point, actually start to rotate around the origin. For example, it the particle is a bullet, and the X-Y plane is a stationary wooden plate around an axis at (0,0), with a slot in it so the bullet hasn't struck the plate yet, then the angular velocity would become significant only once it strikes the edge of the plate. It could then be used, along with other parameters of the plate and bullet, to determine how fast the plate would then begin to rotate about the axis. StuRat (talk) 01:42, 2 January 2012 (UTC)
- Thanks for your post. It would have been a better post had you actually addressed the questions I asked. All but the first sentence is totally out of context. — Fly by Night (talk) 02:30, 2 January 2012 (UTC)
- It is truly heartwarming to see how cordial relations are here on the ref desks this New Year. Borrowing the "bullet" and "plane" concepts from StuRat, considering the x-axis as "up", and changing units a bit, we can see the real-world meaning of the angular velocity in this problem. Consider a ref desk regular, at the origin, who asks a vague question. Another ref desk denizen flies overhead in an ultra-light aircraft (named the X-Y) at a constant altitude of 1 km and speed of 1 km/min such that he is directly overhead at time 0. Prior to this time, our intrepid pilot drops a bomb of an answer, which lands directly on, but fails to satisfy the questioner. Should the disgruntled questioner now wish to snipe at the respondent with a scorching reply of his laser cannon, the computed angular velocity represents the angular rate at which the cannon's aim should be depressed to maintain a lock on its target. -- ToE 08:10, 2 January 2012 (UTC)
- Thanks for your post. It would have been a better post had you actually addressed the questions I asked. All but the first sentence is totally out of context. — Fly by Night (talk) 02:30, 2 January 2012 (UTC)
- It makes perfect sense to talk about the angular velocity about the origin for that particle. You can talk about the angular velocity about any point for any particle. If you chose a point on the line x=1, for example, then the angular velocity of your particle would be zero. One interesting thing you can do with that angular velocity is calculate the angular momentum (it's just mass times distance from the point squared times angular velocity for a single particle). If you do so, you'll find that it's a constant (the angular momentum is decreasing and the distance is increasing and they cancel out - check it for yourself). That will be true for any point you chose to measure it relative to. That's the principle of conservation of angular momentum. The angular momentum of your particle will be constant about any point you chose, including the origin, because there are no forces acting on it (it isn't accelerating). --Tango (talk) 02:10, 2 January 2012 (UTC)
- The angular velocity most certainly is not zero. I have already found the angular velocity ω(t). Please read the article on angular velocity. I gave a series of particular questions, and would appreciate it if people answered them instead of giving a list of random facts. — Fly by Night (talk) 02:30, 2 January 2012 (UTC)
- Your question was pretty vague - you clearly know the definition of angular velocity, and that's all I could really tell you about what it means. Therefore, I thought it would be useful to talk about what angular velocity can be used for in order to give you a feel for how it works and why it still makes sense even if the object isn't moving in a circle. As I explained, although apparently not clearly enough, angular velocity can be calculated relative to any point. You calculated it relative to the origin, but there is nothing special about the origin. If you calculate it relative to a point on the line x=1 then you will find that you get zero. As for your other questions - the angular velocity you have calculated clearly isn't infinitesimal (at t=0, it's 1/2, for instance, which is definitely finite) and there isn't a name for the sum of the linear and angular velocities because that is a meaningless concept (you can only add things if they have the same units and linear velocity has units of length/time while angular velocity has units of angle/time). --Tango (talk) 03:47, 2 January 2012 (UTC)
- I agree with Tango, with the minor correction that the magnitude of the angular momentum is , not . Two additional points: (i) "Infinitesimal angular velocity" is certainly incorrect, but "instantaneous angular velocity" would be correct; (ii) if the particle's velocity vector v makes an angle φ with its position vector r then the magnitude of its instantaneous angular velocity (relative to the origin of the position vector) is v sin φ / r. Gandalf61 (talk) 09:31, 2 January 2012 (UTC)
- Thanks for the correction, I've fixed my post. I did consider looking up that formula to make sure and decided I didn't need to... doh! --Tango (talk) 13:23, 2 January 2012 (UTC)
- I agree with Tango, with the minor correction that the magnitude of the angular momentum is , not . Two additional points: (i) "Infinitesimal angular velocity" is certainly incorrect, but "instantaneous angular velocity" would be correct; (ii) if the particle's velocity vector v makes an angle φ with its position vector r then the magnitude of its instantaneous angular velocity (relative to the origin of the position vector) is v sin φ / r. Gandalf61 (talk) 09:31, 2 January 2012 (UTC)
- Your question was pretty vague - you clearly know the definition of angular velocity, and that's all I could really tell you about what it means. Therefore, I thought it would be useful to talk about what angular velocity can be used for in order to give you a feel for how it works and why it still makes sense even if the object isn't moving in a circle. As I explained, although apparently not clearly enough, angular velocity can be calculated relative to any point. You calculated it relative to the origin, but there is nothing special about the origin. If you calculate it relative to a point on the line x=1 then you will find that you get zero. As for your other questions - the angular velocity you have calculated clearly isn't infinitesimal (at t=0, it's 1/2, for instance, which is definitely finite) and there isn't a name for the sum of the linear and angular velocities because that is a meaningless concept (you can only add things if they have the same units and linear velocity has units of length/time while angular velocity has units of angle/time). --Tango (talk) 03:47, 2 January 2012 (UTC)
- The angular velocity most certainly is not zero. I have already found the angular velocity ω(t). Please read the article on angular velocity. I gave a series of particular questions, and would appreciate it if people answered them instead of giving a list of random facts. — Fly by Night (talk) 02:30, 2 January 2012 (UTC)
Thanks to those of you that answered some of my question. The question of what the sum of linear and angular velocity represents has been left addressed. I think I'll go elsewhere for an answer on that one. — Fly by Night (talk) 14:26, 2 January 2012 (UTC)
- Your question about what the sum of linear and angular velocities represents was answered by Tango. As Tango says above, it is just a number with no physical meaning because the two quantities are denominated in different units and, more fundamentally, they are dimensionally different, so you can't even convert them into the same units before adding them. Gandalf61 (talk) 14:57, 2 January 2012 (UTC)
January 2
In the French Wikipedia, there is given an alternative name for the carbon group: Cristallogène (which would probably be "cristallogens" in English). What is the etymology of this name and why isn't it used in English (I've never seen the term in English)? Double sharp (talk) 02:46, 2 January 2012 (UTC)
- It looks like it means "crystal generating". While carbon and silicon readily form crystals, you don't normally think of metals like tin and lead being crystal generating elements (although they might under some conditions, I suppose). Conversely, many other elements not in that group also form crystals readily, so it seems like a questionable name, to me. StuRat (talk) 03:31, 2 January 2012 (UTC)
- You mean like lead crystal? --Jayron32 04:19, 2 January 2012 (UTC)
- That's not made from just lead, but only a small potion of lead added in. StuRat (talk) 06:17, 2 January 2012 (UTC)
- But pnictogen also doesn't fit very well as a name as metals like bismuth aren't really choking. Double sharp (talk) 14:06, 2 January 2012 (UTC)
- And, you'll note, that lead crystal is mostly silicon, which is in the Carbon group. --Jayron32 21:26, 2 January 2012 (UTC)
John Daniel Titius and John Elert Bode
happy new year
what is the real reason of Titius -Bode low? --Akbarmohammadzade (talk) 04:05, 2 January 2012 (UTC)
- It was an attempt to calculating the orbits of the planets in a predictive manner. It seemed to correctly predict the asteroid belt and uranus, but completely missed Neptune. IIRC, given a 66.7% success rate over a small sample size, it may have been just dumb luck that it gets right what it did. --Jayron32 04:17, 2 January 2012 (UTC)
can we generalize it to dynamic of galaxies?(the main mass separates its sub system automatically in such rule?--Akbarmohammadzade (talk) 04:33, 2 January 2012 (UTC)
- The article is Titius-Bode law. The article does have some theoretical explanations - possibly, the way planets form (collapsing disk of matter) can lead to certain regular powers of distance of planet formation, with orbital resonance perhaps increasing the probability of such spacing. By fitting several planets (Mercury, Venus, Mars, Jupiter, and Saturn), some sort of regular pattern was created for the solar system, which two more planets also happened to fit (and Neptune didn't). Note too that, according to the currently most accepted theory on the formation of the solar system, the Nice model, the Uranus and Neptune were formed closer to the Sun, and migrated outward. The Nice model is still very new, and by no means universally accepted. It was later pointed out that some of the large moons of Jupiter and Saturn also followed regular, though non-Bode distance patterns. This expanded model is termed Dermott's law. Is there really some effects that conspire to give Bode-like planet distances, or is it just dumb luck? Maybe if we can start mapping solar systems of other stars, it will become more clear. Buddy431 (talk) 04:50, 2 January 2012 (UTC)
- We have started mapping solar systems of other stars. We know of 6 planets around Gliese 581 for instance. I'm not sure if they follow any kind of Titus-Bode style law or not, though. --Tango (talk) 13:27, 2 January 2012 (UTC)
- The article is Titius-Bode law. The article does have some theoretical explanations - possibly, the way planets form (collapsing disk of matter) can lead to certain regular powers of distance of planet formation, with orbital resonance perhaps increasing the probability of such spacing. By fitting several planets (Mercury, Venus, Mars, Jupiter, and Saturn), some sort of regular pattern was created for the solar system, which two more planets also happened to fit (and Neptune didn't). Note too that, according to the currently most accepted theory on the formation of the solar system, the Nice model, the Uranus and Neptune were formed closer to the Sun, and migrated outward. The Nice model is still very new, and by no means universally accepted. It was later pointed out that some of the large moons of Jupiter and Saturn also followed regular, though non-Bode distance patterns. This expanded model is termed Dermott's law. Is there really some effects that conspire to give Bode-like planet distances, or is it just dumb luck? Maybe if we can start mapping solar systems of other stars, it will become more clear. Buddy431 (talk) 04:50, 2 January 2012 (UTC)
What is the specific gravity of quartz sand?
...in lbs/square-ft lbs/cubed-ft? A family member needs to know this and I have absolutely no idea how to go about finding this (less a Google search, which didn't turn up any immediate answers). The specific gravity article sort of intimidates me, especially the part about temperature and pressure needing to be specified for "both the sample and the reference". This is beyond me, but apparently someone thinks I'm a genius at science. Should've kept my mouth shut during Christmas dinner! Any help would be great, thanks so much! – Kerαunoςcopia◁galaxies 04:05, 2 January 2012 (UTC)
- As sand consists of particles, the specific gravity might vary by the coarseness/fineness of the particles, but I'm not sure whether the more coarse or the more fine sand would have the greater specific gravity. Bus stop (talk) 04:24, 2 January 2012 (UTC)
- Your units aren't right either. True specific gravity is a unitless number. Sometimes when people say specific gravity, they really mean density, but even there, your units aren't right (should be mass per volume. You have weight per area. You might charitably assume they mean pound (mass), but you still need a per volume, rather than per area). What's the context that you need this for? Buddy431 (talk) 04:33, 2 January 2012 (UTC)
- Yes - lbs/cubic ft would make more sense. Actually, following on from Bus Stop, I think that the shape of the particles will matter more - as will the variation in size. The density of 'sand' will necessarily be less than that of the bulk material it is made up of - quartz rock in this case. As for how much less, this will depend on how efficiently it packs together. You'll also need to specify whether you mean dry sand or wet - wet sand can hold a lot of water. AndyTheGrump (talk) 04:41, 2 January 2012 (UTC)
- (edit conflict)Specific gravity is a unitless ratio between the density of the comparing substance to a reference substance (usualy water,) as in how many times heavier is substance A than water at some temperature and pressure. Quartz sand should ideally be composed of crushed quartz; the specific gravity is 2.65 from the quartz article. However, it could be different due to impurities. Plasmic Physics (talk) 04:57, 2 January 2012 (UTC)
- If you want to include the voids between grains, then a simple experiment would be to simply measure the volume taken up by a pound of water, and a pound of quartz sand, and divide the first value by second. Plasmic Physics (talk) 05:05, 2 January 2012 (UTC)
- A Google search on [density sand] yields tables with a wide range of values -- all the way from 90 lb/ft3 to 100 lb/ft3 for dry loose sand up to 120 lb/ft3 to 130 lb/ft3 for wet packed sand. If you truly want an answer in lbs/square-ft, then you are seeking the areal density, and you need to multiply by the desired thickness. For instance, a four inch layer of damp packed sand with a density of 120 lb/ft3 has an areal density of 40 lb/ft2. If you are seeking precise numbers, you will need to provide more information in your question. -- ToE 05:38, 2 January 2012 (UTC)
- (E.C.) Also note that many of the density tables you find online give metric values, and while you can convert directly, an understanding of specific gravity can help you do it in your head. Unless you are interested in a great deal of precision, don't worry about the finer details in our article which threw you off; just think of specific gravity as the ratio of density of the substance in question to the density of water. Thus if a table states that wet, packed sand has a specific gravity of 2.082, then you know that it is 2.082 times more dense than water. The density of water is about 62.4 lb/ft3, so just multiply to find that the density of wet, packed sand is 130 lb/ft3 . Many of the tables you will find will give densities in metric units, but don't despair, as you can read the specific gravity directly from such figures. This is because the metric system was set up so that the density of water is 1 g/cm3, which is the same as 1 g/ml, or 1000 kg/m3, or 1 metric ton per cubic meter. Thus, when you see a table which gives the density of wet, packed sand as being "2082 kg/cu.m", you know that is 2.082 times as dense as water, and is thus the same as 130 lb/ft3. Does that help? -- ToE 06:02, 2 January 2012 (UTC)
- Oops, I meant cubed-feet (fixed). I knew there'd be more to this than meets the eye! : ) I will pass on the information. Thanks so much for everyone's help! Sorry for the error again. – Kerαunoςcopia◁galaxies 05:43, 2 January 2012 (UTC)
- I just reinserted the original "lbs/square-ft" into your question, but
struck out, so that future readers of the archives will understand why some of the answers were discussing units the way they were. Hope you don't mind. -- ToE 06:09, 2 January 2012 (UTC)
- I just reinserted the original "lbs/square-ft" into your question, but
- Oops, I meant cubed-feet (fixed). I knew there'd be more to this than meets the eye! : ) I will pass on the information. Thanks so much for everyone's help! Sorry for the error again. – Kerαunoςcopia◁galaxies 05:43, 2 January 2012 (UTC)
health education
what is health education? — Preceding unsigned comment added by 85.15.40.77 (talk) 07:24, 2 January 2012 (UTC)
- Just what it sounds like, education about human health. Some basic biology is usually included, including sex education. These days, more emphasis on a proper diet and exercise might also be included, in the hopes of fighting obesity. StuRat (talk) 07:27, 2 January 2012 (UTC)
- We have an article with just that name - Health education. It should help. HiLo48 (talk) 07:29, 2 January 2012 (UTC)
Mammoth
How long will it be before somebody seeks to make one inside an elephant? Kittybrewster ☎ 16:51, 2 January 2012 (UTC)
- Who says they aren't already trying [9] :) IRWolfie- (talk) 16:55, 2 January 2012 (UTC)
- The Pleistocene Park effort is related to that (I think it's the same people). -- Finlay McWalterჷTalk 21:09, 2 January 2012 (UTC)
- Yep, it's a joint effort by Russian and Japanese scientists. ETA is 5 years.-- Obsidi♠n Soul 21:17, 2 January 2012 (UTC)
Higher derivatives of displacement w.r.t. time
The Wikipedia article on displacement lists several names for some higher derivatives of displacement w.r.t. time: snap, crackle, pop, lock, and drop (together with their synonyms). I've never seen these names before but I hesitate to declare them unestablished. Does anyone know how standard these terms are, and whether they meet Wikipedia's criteria for inclusion in the article? — Preceding unsigned comment added by 173.49.10.51 (talk) 20:30, 2 January 2012 (UTC)
- Unhelpfully, that information claimed to referenced to wearcam.org, which is (really really obviously) an old mirror of Wikipedia itself, and thus isn't a reliable source. -- Finlay McWalterჷTalk 20:46, 2 January 2012 (UTC)
- Jounce does cite a source for a couple of them, but the citation calls their use "facetiously" -- Finlay McWalterჷTalk 20:48, 2 January 2012 (UTC)
- This article states, "Another less serious suggestion is snap (symbol s), crackle (symbol c) and pop (symbol p) for the 4th, 5th and 6th derivatives respectively. Higher derivatives do not yet have names because they do not come up very often." Based on this, I'd be inclined to just replace the 4th-6th equations with a sentence to that effect and drop the higher ones entirely. Clarityfiend (talk) 20:55, 2 January 2012 (UTC)
- Searching Google for that phrase "In the UK jolt has sometimes been used instead of jerk" finds it used in all kinds of places; it's not clear what the real source is, and for us to give it credence as a ref we'd need to know who originally wrote it, and whether they're a reliable source. That specific page says it comes from someone called "Philip Gibbs", but who is that? It's on John Baez' website, which is a good sign, but it's not from Baez himself, so it's not a reliable source yet. -- Finlay McWalterჷTalk 21:06, 2 January 2012 (UTC)
- Google scholar finds hits for 'jounce acceleration', and from memory, I think an Open university programme referred to 'joggle and jounce' in regard to fairground ride designs. AndyTheGrump (talk) 20:58, 2 January 2012 (UTC)
- More - A Google Scolar search for 'jounce fairground' [10] finds an Oxford Journals article [11] that apparently contains the following: "Students can use a computer algebra system, such as Maple, to experiment with different fairground designs, eg to determine the jerk and jounce (third and fourth derivatives) of a track or find any track with a specified jerk and jounce...". I've not got access to the paper, but it looks convincing. AndyTheGrump (talk) 21:39, 2 January 2012 (UTC)
Wikipedia is not an indiscriminate list of any term anybody ever used to describe anything. Those names are uncommon. I have removed them from the article, and replaced the section with a discussion, including cited sources. Nimur (talk) 21:48, 2 January 2012 (UTC)
immunolin
Know anything about "Immunolin"? It's a product that's supposed to help boost the immune system.




