NF-κB

Template:FixBunching NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) is a protein complex that acts as a transcription factor. NF-κB is found in almost all animal cell types and is involved in cellular responses to stimuli such as stress, cytokines, free radicals, ultraviolet irradiation, oxidized LDL, and bacterial or viral antigens.[1][2][3][4][5] NF-κB plays a key role in regulating the immune response to infection. Consistent with this role, incorrect regulation of NF-κB has been linked to cancer, inflammatory and autoimmune diseases, septic shock, viral infection, and improper immune development. NF-κB has also been implicated in processes of synaptic plasticity and memory.[6]
Discovery
NF-κB was first discovered in the lab of Nobel Prize laureate David Baltimore via its interaction with an 11-base pair sequence in the immunoglobulin light-chain enhancer in B cells.[7]
Structure
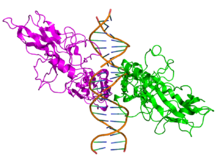
All proteins of the NF-κB family share a Rel homology domain in their N-terminus. A subfamily of NF-κB proteins, including RelA, RelB, and c-Rel, have a transactivation domain in their C-termini. In contrast, the NF-κB1 and NF-κB2 proteins are synthesized as large precursors, p105, and p100, which undergo processing to generate the mature NF-κB subunits, p50 and p52, respectively. The processing of p105 and p100 is mediated by the ubiquitin/proteasome pathway and involves selective degradation of their C-terminal region containing ankyrin repeats. Whereas the generation of p52 from p100 is a tightly-regulated process, p50 is produced from constitutive processing of p105.[8][9]
Members
NF-κB family members share structural homology with the retroviral oncoprotein v-Rel, resulting in their classification as NF-κB/Rel proteins.[1]
There are five proteins in the mammalian NF-κB family:[10]
| Class | Protein | Aliases | Gene |
|---|---|---|---|
| Class I | NF-κB1 | p105 → p50 | NFKB1 |
| NF-κB2 | p100 → p52 | NFKB2 | |
| Class II | RelA | p65 | RELA |
| RelB | RELB | ||
| c-Rel | REL |
Below are the five human NF-κB family members:
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In addition, there are NF-κB proteins in invertebrates, such as the fruit fly Drosophila, sea urchins, sea anemones, and sponges.[11]
Activation
Part of NF-κB's importance in regulating cellular responses is that it belongs to the category of "rapid-acting" primary transcription factors, i.e., transcription factors that are present in cells in an inactive state and do not require new protein synthesis to be activated (other members of this family include transcription factors such as c-Jun, STATs, and nuclear hormone receptors). This allows NF-κB to act as a "first responder" to harmful cellular stimuli. Stimulation of a wide variety of cell-surface receptors, such as RANK, TNFR, leads directly to NF-κB activation and fairly rapid changes in gene expression.[1]
Many bacterial products can activate NF-κB. The identification of Toll-like receptors (TLRs) as specific pattern recognition molecules and the finding that stimulation of TLRs leads to activation of NF-κB improved our understanding of how different pathogens activate NF-κB. For example, studies have identified TLR4 as the receptor for the LPS component of Gram-Negative bacteria.[12] TLRs are key regulators of both innate and adaptive immune responses.[13]
Unlike RelA, RelB, and c-Rel, the p50 and p52 NF-κB subunits do not contain transactivation domains in their C terminal halves. Nevertheless, the p50 and p52 NF-κB members play critical roles in modulating the specificity of NF-κB function. Although homodimers of p50 and p52 are, in general, repressors of κB site transcription, both p50 and p52 participate in target gene transactivation by forming heterodimers with RelA, RelB, or c-Rel.[14] In addition, p50 and p52 homodimers also bind to the nuclear protein Bcl-3, and such complexes can function as transcriptional activators.[15][16][17]
Inhibition
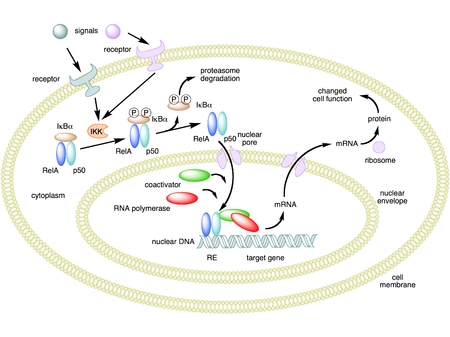
In unstimulated cells, the NF-κB dimers are sequestered in the cytoplasm by a family of inhibitors, called IκBs (Inhibitor of κB), which are proteins that contain multiple copies of a sequence called ankyrin repeats. By virtue of their ankyrin repeat domains, the IκB proteins mask the nuclear localization signals (NLS) of NF-κB proteins and keep them sequestered in an inactive state in the cytoplasm.[18]
IκBs are a family of related proteins that have an N-terminal regulatory domain, followed by six or more ankyrin repeats and a PEST domain near their C terminus. Although the IκB family consists of IκBα, IκBβ, IκBγ, IκBε, and Bcl-3, the best-studied and major IκB protein is IκBα. Due to the presence of ankyrin repeats in their C-terminal halves, p105 and p100 also function as IκB proteins. Of all the IκB members, IκBγ is unique in that it is synthesized from the nF-kb1 gene using an internal promoter, thereby resulting in a protein that is identical to the C-terminal half of p105.[19] The c-terminal half of p100, that is often referred to as IκBδ, also functions as an inhibitor.[20][21] IκBδ degradation in response to developmental stimuli, such as those transduced through LTβR, potentiate NF-κB dimer activation in a NIK dependent non-canonical pathway.[20][22]
Activation of the NF-κB is initiated by the signal-induced degradation of IκB proteins. This occurs primarily via activation of a kinase called the IκB kinase (IKK). IKK is composed of a heterodimer of the catalytic IKK alpha and IKK beta subunits and a "master" regulatory protein termed NEMO (NF-κB essential modulator) or IKK gamma. When activated by signals, usually coming from the outside of the cell, the IκB kinase phosphorylates two serine residues located in an IκB regulatory domain. When phosphorylated on these serines (e.g., serines 32 and 36 in human IκBα), the IκB inhibitor molecules are modified by a process called ubiquitination, which then leads them to be degraded by a cell structure called the proteasome.
With the degradation of the IκB inhibitor, the NF-κB complex is then freed to enter the nucleus where it can 'turn on' the expression of specific genes that have DNA-binding sites for NF-κB nearby. The activation of these genes by NF-κB then leads to the given physiological response, for example, an inflammatory or immune response, a cell survival response, or cellular proliferation. NF-κB turns on expression of its own repressor, IκBα. The newly synthesized IκBα then re-inhibits NF-κB and, thus, forms an auto feedback loop, which results in oscillating levels of NF-κB activity.[23] In addition, several viruses, including the AIDS virus HIV, have binding sites for NF-κB that controls the expression of viral genes, which in turn contribute to viral replication or viral pathogenicity. In the case of HIV-1, activation of NF-κB may, at least in part, be involved in activation of the virus from a latent, inactive state.[24] YopJ is a factor secreted by Yersinia pestis, the causative agent of plague, that prevents the ubiquitination of IκB. This causes this pathogen to effectively inhibit the NF-κB pathway and thus block the immune response of a human infected with Yersinia.[25]
Clinical significance
NF-κB is widely used by eukaryotic cells as a regulator of genes that control cell proliferation and cell survival. As such, many different types of human tumors have misregulated NF-κB: that is, NF-κB is constitutively active. Active NF-κB turns on the expression of genes that keep the cell proliferating and protect the cell from conditions that would otherwise cause it to die via apoptosis. Defects in NF-κB results in increased susceptibility to apoptosis leading to increased cell death. This is because NF-κB regulates anti-apoptotic genes especially the TRAF1 and TRAF2 and thereby checks the activities of the caspase family of enzymes which are central to most apoptotic processes.[26]
In tumor cells, NF-κB is active either due to mutations in genes encoding the NF-κB transcription factors themselves or in genes that control NF-κB activity (such as IκB genes); in addition, some tumor cells secrete factors that cause NF-κB to become active. Blocking NF-κB can cause tumor cells to stop proliferating, to die, or to become more sensitive to the action of anti-tumor agents. Thus, NF-κB is the subject of much active research among pharmaceutical companies as a target for anti-cancer therapy.[27]
Because NF-κB controls many genes involved in inflammation, it is not surprising that NF-κB is found to be chronically active in many inflammatory diseases, such as inflammatory bowel disease, arthritis, sepsis, asthma, among others. Many natural products (including anti-oxidants) that have been promoted to have anti-cancer and anti-inflammatory activity have also been shown to inhibit NF-κB. There is a controversial US patent (US patent 6,410,516)[28] that applies to the discovery and use of agents that can block NF-κB for therapeutic purposes. This patent is involved in several lawsuits, including Ariad v. Lilly. Recent work by Karin,[29] Ben-Neriah[30] and others has highlighted the importance of the connection between NF-κB, inflammation, and cancer, and underscored the value of therapies that regulate the activity of NF-κB.[31]
As a drug target
Aberrant activation of NF-κB is frequently observed in many cancers. Furthermore suppression of NF-κB limits the proliferation of cancer cells. In addition, NF-κB is a key player in the inflammatory response. Hence methods of inhibiting NF-κB signaling has potential therapeutic application in cancer and inflammatory diseases.[32][33]
The discovery that activation of NF-κB nuclear translocation can be separated from the elevation of oxidant stress[34] gives an important hint to the development of strategies for NF-κB inhibition.
Disulfiram, olmesartan and dithiocarbamates can inhibit the nuclear factor-κB (NF-κB) signaling cascade.[35]
Signaling in immunity
NF-kB is a major transcription factor that regulates genes responsible for both the innate and adaptive immune response. Upon activation of either the T- or B-cell receptor, NF-kB becomes activated through distinct signaling components. Upon ligation of the T-cell receptor, an adaptor molecule, ZAP70 is recruited via its SH2 domain to the cytoplasmic side of the receptor. ZAP70 helps recruit both LCK and PLC-γ, which causes activation of PKC. Through a cascade of phosphorylation events, the kinase complex is activated and NF-kB is able to enter the nucleus to upregulate genes involved in T-cell development, maturation, and proliferation.[36]
Conserved in evolution
NF-kB is found in a number of simple animals as well. These include cnidarians (such as sea anemones and coral), porifera (sponges), and insects (such as moths, mosquitoes, and fruitflies). The sequencing of the genomes of the mosquitoes A. aegypti and A. gambiae, and the fruitfly D. melanogaster has allowed comparative genetic and evolutionary studies on NF-kB. In those insect species, activation of NF-kB is triggered by the Toll pathway (which evolved independently in insects and mammals) and by the Imd pathway.[37]
NF-κB in neurons
The role of NF-κB in neurons of the central nervous system is controversial. Several studies have claimed that the transcription factor is either constitutively active in neurons or activated by excitatory amino acid neurotransmitters or both. However, most of the suggestive data are imprecise, either depending on assays of whole-tissue homogenates (which include other cell types) or reporting only nuclear translocation without a measure of gene transactivation. Studies done with highly purified neurons (i.e., <1% contamination by other cell types) show no activation of NF-κB in response to previously reported agonists such as glutamate[38].
There is a rational argument to be made for restricting this transcription factor in neurons: NF-κB activity can result in expression of class I major histocompatibility complex (MHC I), targeting the cell for removal by cytotoxic T-cells. For the finite population of post-mitotic neurons, this would be maladaptive. Protection of neurons from T-cell-mediated killing via suppression of NF-κB, and thus MHC I, may be one factor in making neurons permissive hosts for viruses.
See also
References
- ^ a b c d e Gilmore TD (2006). "Introduction to NF-κB: players, pathways, perspectives". Oncogene. 25 (51): 6680–4. doi:10.1038/sj.onc.1209954. PMID 17072321.
- ^ a b c Brasier AR (2006). "The NF-κB regulatory network". Cardiovasc. Toxicol. 6 (2): 111–30. doi:10.1385/CT:6:2:111. PMID 17303919.
- ^ a b c Perkins ND (2007). "Integrating cell-signalling pathways with NF-κB and IKK function". Nat. Rev. Mol. Cell Biol. 8 (1): 49–62. doi:10.1038/nrm2083. PMID 17183360.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Gilmore TD (1999). "The Rel/NF-κB signal transduction pathway: introduction". Oncogene. 18 (49): 6842–4. doi:10.1038/sj.onc.1203237. PMID 10602459.
- ^ Tian B, Brasier AR (2003). "Identification of a nuclear factor κ B-dependent gene network". Recent Prog. Horm. Res. 58: 95–130. doi:10.1210/rp.58.1.95. PMID 12795416.
- ^ Albensi BC, Mattson MP (2000). "Evidence for the involvement of TNF and NF-κB in hippocampal synaptic plasticity". Synapse. 35 (2): 151–9. doi:10.1002/(SICI)1098-2396(200002)35:2<151::AID-SYN8>3.0.CO;2-P. PMID 10611641.
- ^ Sen R, Baltimore D (1986). "Multiple nuclear factors interact with the immunoglobulin enhancer sequences". Cell. 46 (5): 705–16. doi:10.1016/0092-8674(86)90346-6. PMID 3091258.
- ^ Karin M, Ben-Neriah Y (2000). "Phosphorylation meets ubiquitination: the control of NF-κB activity". Annu. Rev. Immunol. 18: 621–63. doi:10.1146/annurev.immunol.18.1.621. PMID 10837071.
- ^ Senftleben U, Cao Y, Xiao G, Greten FR, Krähn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M (2001). "Activation by IKKalpha of a second, evolutionary conserved, NF-κB signaling pathway". Science. 293 (5534): 1495–9. doi:10.1126/science.1062677. PMID 11520989.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Nabel GJ, Verma IM (1993). "Proposed NF-κB/IκB family nomenclature". Genes Dev. 7 (11): 2063. PMID 8224837.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Ghosh S, May MJ, Kopp EB (1998). "NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses". Annu. Rev. Immunol. 16: 225–60. doi:10.1146/annurev.immunol.16.1.225. PMID 9597130.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Doyle SL, O'Neill LA (2006). "Toll-like receptors: from the discovery of NFκB to new insights into transcriptional regulations in innate immunity". Biochem. Pharmacol. 72 (9): 1102–13. doi:10.1016/j.bcp.2006.07.010. PMID 16930560.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Hayden MS, West AP, Ghosh S (2006). "NF-κB and the immune response". Oncogene. 25 (51): 6758–80. doi:10.1038/sj.onc.1209943. PMID 17072327.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Li Q, Verma IM (2002). "NF-κB regulation in the immune system". Nat. Rev. Immunol. 2 (10): 725–34. doi:10.1038/nri910. PMID 12360211.
- ^ Fujita T, Nolan GP, Liou HC, Scott ML, Baltimore D (1993). "The candidate proto-oncogene bcl-3 encodes a transcriptional coactivator that activates through NF-κB p50 homodimers". Genes Dev. 7 (7B): 1354–63. doi:10.1101/gad.7.7b.1354. PMID 8330739.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Franzoso G, Bours V, Park S, Tomita-Yamaguchi M, Kelly K, Siebenlist U (1992). "The candidate oncoprotein Bcl-3 is an antagonist of p50/NF-κB-mediated inhibition". Nature. 359 (6393): 339–42. doi:10.1038/359339a0. PMID 1406939.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bours V, Franzoso G, Azarenko V, Park S, Kanno T, Brown K, Siebenlist U (1993). "The oncoprotein Bcl-3 directly transactivates through κ B motifs via association with DNA-binding p50B homodimers". Cell. 72 (5): 729–39. doi:10.1016/0092-8674(93)90401-B. PMID 8453667.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jacobs MD, Harrison SC (1998). "Structure of an IκBalpha/NF-κB complex". Cell. 95 (6): 749–58. doi:10.1016/S0092-8674(00)81698-0. PMID 9865693.
- ^ Inoue J, Kerr LD, Kakizuka A, Verma IM (1992). "IκB gamma, a 70 kd protein identical to the C-terminal half of p110 NF-κB: a new member of the IκB family". Cell. 68 (6): 1109–20. doi:10.1016/0092-8674(92)90082-N. PMID 1339305.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Basak S, Kim H, Kearns JD, Tergaonkar V, O'dea E, Werner SL, Benedict CA, Ware CF, Ghosh G, Verma IM, Hoffmann A (2007). "A fourth IκB protein within the NF-κB signaling module". Cell. 128 (2): 369–81. doi:10.1016/j.cell.2006.12.033. PMID 17254973.
{{cite journal}}: CS1 maint: multiple names: authors list (link). - ^ Dobrzanski P, Ryseck RP, Bravo R (1995). "Specific inhibition of RelB/p52 transcriptional activity by the C-terminal domain of p100". Oncogene. 10 (5): 1003–7. PMID 7898917.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lo JC, Basak S, James ES, Quiambo RS, Kinsella MC, Alegre ML, Weih F, Franzoso G, Hoffmann A, Fu YX (2006). "Coordination between NF-κB family members p50 and p52 is essential for mediating LTbetaR signals in the development and organization of secondary lymphoid tissues". Blood. 107 (3): 1048–55. doi:10.1182/blood-2005-06-2452. PMID 16195333.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Nelson DE, Ihekwaba AE, Elliott M, Johnson JR, Gibney CA, Foreman BE, Nelson G, See V, Horton CA, Spiller DG, Edwards SW, McDowell HP, Unitt JF, Sullivan E, Grimley R, Benson N, Broomhead D, Kell DB, White MR (2004). "Oscillations in NF-κB signaling control the dynamics of gene expression". Science. 306 (5696): 704–8. doi:10.1126/science.1099962. PMID 15499023.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hiscott J, Kwon H, Génin P (2001). "Hostile takeovers: viral appropriation of the NF-κB pathway". J. Clin. Invest. 107 (2): 143–51. doi:10.1172/JCI11918. PMC 199181. PMID 11160127.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Adkins I, Schulz S, Borgmann S, Autenrieth IB, Gröbner S (2008). "Differential roles of Yersinia outer protein P-mediated inhibition of nuclear factor-κB in the induction of cell death in dendritic cells and macrophages". J. Med. Microbiol. 57 (Pt 2): 139–44. doi:10.1099/jmm.0.47437-0. PMID 18201977.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Sheikh MS, Huang Y (2003). "Death receptor activation complexes: it takes two to activate TNF receptor 1". Cell Cycle. 2 (6): 550–2. PMID 14504472.
- ^ Escárcega RO, Fuentes-Alexandro S, García-Carrasco M, Gatica A, Zamora A (2007). "The transcription factor nuclear factor-κB and cancer". Clinical Oncology (Royal College of Radiologists (Great Britain)). 19 (2): 154–61. doi:10.1016/j.clon.2006.11.013. PMID 17355113.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ US patent 6410516, Baltimore D; Sen R; Sharp PA; Singh H; Staudt L; Lebowitz JH; Baldwin Jr AS; Clerc RG; Corcoran LM; Baeuerle PA; Lenardo MJ; Fan C-M; Maniatis TPD, "Nuclear factors associated with transcriptional regulation", issued 2002-06-25
- ^ Karin M (2008). "The IκB kinase - a bridge between inflammation and cancer". Cell Res. 18 (3): 334–42. doi:10.1038/cr.2008.30. PMID 18301380.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Pikarsky E, Ben-Neriah Y (2006). "NF-κB inhibition: a double-edged sword in cancer?". Eur. J. Cancer. 42 (6): 779–84. doi:10.1016/j.ejca.2006.01.011. PMID 16530406.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Mantovani A, Marchesi F, Portal C, Allavena P, Sica A (2008). "Linking inflammation reactions to cancer: novel targets for therapeutic strategies". Adv. Exp. Med. Biol. 610: 112–27. PMID 18593019.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Garg A, Aggarwal BB (2002). "Nuclear transcription factor-kappaB as a target for cancer drug development". Leukemia. 16 (6): 1053–68. doi:10.1038/sj.leu.2402482. PMID 12040437.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Sethi G, Sung B, Aggarwal BB (2008). "Nuclear factor-kappaB activation: from bench to bedside". Exp. Biol. Med. (Maywood). 233 (1): 21–31. doi:10.3181/0707-MR-196. PMID 18156302.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Vlahopoulos S, Boldogh I, Casola A, Brasier AR (1999). "Nuclear factor-κB-dependent induction of interleukin-8 gene expression by tumor necrosis factor alpha: evidence for an antioxidant sensitive activating pathway distinct from nuclear translocation". Blood. 94 (6): 1878–89. PMID 10477716.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Cvek B, Dvorak Z (2007). "Targeting of nuclear factor-κB and proteasome by dithiocarbamate complexes with metals". Curr. Pharm. Des. 13 (30): 3155–67. doi:10.2174/138161207782110390. PMID 17979756.
- ^ Livolsi A, Busuttil V, Imbert V, Abraham RT, Peyron JF (2001). "Tyrosine phosphorylation-dependent activation of NF-κB. Requirement for p56 LCK and ZAP-70 protein tyrosine kinases". Eur. J. Biochem. 268 (5): 1508–15. doi:10.1046/j.1432-1327.2001.02028.x. PMID 11231305.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Waterhouse RM, Kriventseva EV, Meister S, Xi Z, Alvarez KS, Bartholomay LC, Barillas-Mury C, Bian G, Blandin S, Christensen BM, Dong Y, Jiang H, Kanost MR, Koutsos AC, Levashina EA, Li J, Ligoxygakis P, Maccallum RM, Mayhew GF, Mendes A, Michel K, Osta MA, Paskewitz S, Shin SW, Vlachou D, Wang L, Wei W, Zheng L, Zou Z, Severson DW, Raikhel AS, Kafatos FC, Dimopoulos G, Zdobnov EM, Christophides GK (2007). "Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes". Science. 316 (5832): 1738–43. doi:10.1126/science.1139862. PMID 17588928.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Massa PT, Aleyasin H, Park DS, Mao X, Barger SW (2006). "NFκB in neurons? The uncertainty principle in neurobiology". J. Neurochem. 97: 607-618.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
- NF-kappa+B at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Sankar Ghosh (2006). Handbook of Transcription Factor NF-κB. Boca Raton: CRC. ISBN 0-8493-2794-6.
- Thomas D Gilmore. "The Rel/NF-κB Signal Transduction Pathway". Boston University. Retrieved 2007-12-02.