Peptide nucleic acid
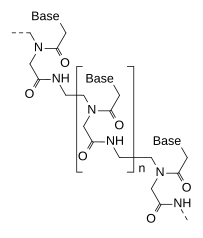
Peptide nucleic acid (PNA) is an artificially synthesized polymer similar to DNA or RNA and is used in biological research and medical treatments. PNA is not known to occur naturally.
DNA and RNA have a deoxyribose and ribose sugar backbone, respectively, whereas PNA's backbone is composed of repeating N-(2-aminoethyl)-glycine units linked by peptide bonds. The various purine and pyrimidine bases are linked to the backbone by methylene carbonyl bonds. PNAs are depicted like peptides, with the N-terminus at the first (left) position and the C-terminus at the right.[1]
Since the backbone of PNA contains no charged phosphate groups, the binding between PNA/DNA strands is stronger than between DNA/DNA strands due to the lack of electrostatic repulsion. Early experiments with homopyrimidine strands (strands consisting of only one repeated pyrimidine base) have shown that the Tm ("melting" temperature) of a 6-base thymine PNA/adenine DNA double helix was 31 °C in comparison to an equivalent 6-base DNA/DNA duplex that denatures at a temperature less than 10 °C. Mixed base PNA molecules are true mimics of DNA molecules in terms of base-pair recognition. PNA/PNA binding is stronger than PNA/DNA binding.
Synthetic peptide nucleic acid oligomers have been used in recent years in molecular biology procedures, diagnostic assays and antisense therapies. Due to their higher binding strength it is not necessary to design long PNA oligomers for use in these roles, which usually require oligonucleotide probes of 20–25 bases. The main concern of the length of the PNA-oligomers is to guarantee the specificity. PNA oligomers also show greater specificity in binding to complementary DNAs, with a PNA/DNA base mismatch being more destabilizing than a similar mismatch in a DNA/DNA duplex. This binding strength and specificity also applies to PNA/RNA duplexes. PNAs are not easily recognized by either nucleases or proteases, making them resistant to enzyme degradation. PNAs are also stable over a wide pH range. Though an unmodified PNA cannot readily cross cell membranes to enter the cytosol, covalently coupling a cell penetrating peptide to a PNA can improve cytosolic delivery.
It has been hypothesized that the earliest life on Earth may have used PNA as a genetic material due to its extreme robustness, simpler formation and possible spontaneous polymerization at 100°C[2] (while water at standard pressure boils at this temperature, water at high pressure—as in deep ocean—boil at higher temperatures). If this is so, life evolved to a DNA/RNA-based system only at a later stage[3] [4]. Evidence for this PNA world hypothesis is however far from conclusive. See RNA world hypothesis for related information.[5]
See also
References
- ^ Egholm, M., Buchardt, O., Christensen, L., Behrens, C., Freier, S.M., Driver, D. A., Berg, R.H., Kim, S.K., Nordén, B. and Nielsen, P.E. PNA Hybridizes to Complementary Oligonucleotides Obeying the Watson-Crick Hydrogen Bonding Rules. (1993) Nature, 365, 566-568.
- ^ Wittung, P., Nielsen P.E., Buchardt, Ole., Egholm, M. and Nordén, B. DNA-like Double Helix formed by Peptide Nucleic Acid. (1994) Nature, 368, 561-563.
- ^ Nelson, K.E., Levy, M., and Miller, S.L. Peptide nucleic acids rather than RNA may have been the first genetic molecule (2000) Proc. Natl. Acad. Sci. USA 97, 3868–3871.
- ^ Alberts, Johnson, Lewis, Raff, Roberts and Walter, Molecular Biology of the Cell, 4th Edition, Routledge, March, 2002, ISBN 0-8153-3218-1.
- ^ Zimmer, C; On the Origin of Life on Earth, Science 9 January 2009, Vol. 323. no. 5911, pp. 198 - 199, DOI: 10.1126/science.323.5911.198
