Dioxygen difluoride

| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Dioxygen difluoride | |||
| Systematic IUPAC name
Fluorooxygen hypofluorite | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | FOOF | ||
| ChEBI | |||
| ChemSpider | |||
| 1570 | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| O 2F 2 | |||
| Molar mass | 69.996 g·mol−1 | ||
| Appearance | orange as a solid red as a liquid | ||
| Density | 1.45 g/cm3 (at b.p.) | ||
| Melting point | −154 °C (−245 °F; 119 K) | ||
| Boiling point | −57 °C (−71 °F; 216 K) extrapolated | ||
| Solubility in other solvents | decomposes | ||
| Thermochemistry | |||
Heat capacity (C)
|
62.1 J/(mol·K) | ||
Std molar
entropy (S⦵298) |
277.2 J/(mol·K) | ||
Std enthalpy of
formation (ΔfH⦵298) |
19.2 kJ/mol | ||
Gibbs free energy (ΔfG⦵)
|
58.2 kJ/mol | ||
| Related compounds | |||
Related compounds
|
|||
| Hazards | |||
| GHS labelling: | |||
   
| |||
| Danger | |||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Dioxygen difluoride is a compound of fluorine and oxygen with the molecular formula O2F2. It can exist as an orange-red colored solid which melts into a red liquid at −163 °C (110 K). It is an extremely strong oxidant and decomposes into oxygen and fluorine even at −160 °C (113 K) at a rate of 4% per day — its lifetime at room temperature is thus extremely short.[1] Dioxygen difluoride reacts vigorously with nearly every chemical it encounters (including ordinary ice) leading to its onomatopoeic nickname FOOF (a play on its chemical structure and its explosive tendencies).[2]
Preparation
Dioxygen difluoride can be obtained by subjecting a 1:1 mixture of gaseous fluorine and oxygen at low pressure (7–17 mmHg (0.9–2.3 kPa) is optimal) to an electric discharge of 25–30 mA at 2.1–2.4 kV.[3]
A similar method was used for the first synthesis by Otto Ruff in 1933.[4] Another synthesis involves mixing O
2 and F
2 in a stainless steel vessel cooled to −196 °C (77.1 K), followed by exposing the elements to 3 MeV bremsstrahlung for several hours. A third method requires heating a mix of fluorine and oxygen to 700 °C (1,292 °F), and then rapidly cooling it using liquid oxygen.[5] All of these methods involve synthesis according to the equation
- O
2 + F
2 → O
2F
2
It also arises from the thermal decomposition of ozone difluoride:[6]
- 2 O
3F
2 → 2 O
2F
2 + O
2
Structure and properties
In O
2F
2, oxygen is assigned the unusual oxidation state of +1. In most of its other compounds, oxygen has an oxidation state of −2.
The structure of dioxygen difluoride resembles that of hydrogen peroxide, H
2O
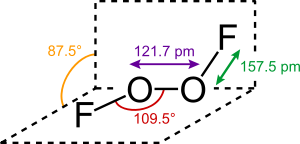
2, in its large dihedral angle, which approaches 90° and C2 symmetry. This geometry conforms with the predictions of VSEPR theory.
The bonding within dioxygen difluoride has been the subject of considerable speculation, particularly because of the very short O−O distance and the long O−F distances. The O−O bond length is within 2 pm of the 120.7 pm distance for the O=O double bond in the dioxygen molecule, O
2. Several bonding systems have been proposed to explain this, including an O−O triple bond with O−F single bonds destabilised and lengthened by repulsion between the lone pairs on the fluorine atoms and the π orbitals of the O−O bond.[7] Repulsion involving the fluorine lone pairs is also responsible for the long and weak covalent bonding in the fluorine molecule.
Computational chemistry indicates that dioxygen difluoride has an exceedingly high barrier to rotation of 81.17 kJ/mol around the O−O bond (in hydrogen peroxide the barrier is 29.45 kJ/mol); this is close to the O−F bond disassociation energy of 81.59 kJ/mol.[8]
The 19F NMR chemical shift of dioxygen difluoride is 865 ppm, which is by far the highest chemical shift recorded for a fluorine nucleus, thus underlining the extraordinary electronic properties of this compound. Despite its instability, thermochemical data for O
2F
2 have been compiled.[9]
Reactivity
The compound readily decomposes into oxygen and fluorine. Even at a temperature of −160 °C (113 K), 4% decomposes each day[1] by this process:
- O
2F
2 → O
2 + F
2
The other main property of this unstable compound is its oxidizing power, although most experimental reactions have been conducted near −100 °C (173 K).[10] Several experiments with the compound resulted in a series of fires and explosions. Some of the compounds that produced violent reactions with O
2F
2 include ethyl alcohol, methane, ammonia, and even water ice.[10]
With BF
3 and PF
5, it gives the corresponding dioxygenyl salts:[1][11]
- 2 O
2F
2 + 2 PF
5 → 2 [O
2]+
[PF
6]−
+ F
2
Uses
The compound currently has no practical applications, but has been of theoretical interest. Los Alamos National Laboratory used it to synthesize plutonium hexafluoride at unprecedentedly low temperatures, which was significant because previous methods for preparation needed temperatures so high that the plutonium hexafluoride created would decompose rapidly.[12]
See also
References
- ^ a b c Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. Academic Press. ISBN 978-0-12-352651-9.
- ^ Lowe, Derek (2010-02-23). "Things I Won't Work With: Dioxygen Difluoride". www.science.org. Retrieved 2022-05-26.
- ^ Kwasnik, W. (1963). "Dioxygen Difluoride". In Brauer, G. (ed.). Handbook of Preparative Inorganic Chemistry. Vol. 1 (2nd ed.). NY: Academic Press. p. 162.
- ^ Ruff, O.; Mensel, W. (1933). "Neue Sauerstofffluoride: O
2F
2 und OF". Zeitschrift für anorganische und allgemeine Chemie. 211 (1–2): 204–208. doi:10.1002/zaac.19332110122. - ^ Mills, Thomas (1991). "Direct synthesis of liquid-phase dioxygen difluoride". Journal of Fluorine Chemistry. 52 (3): 267–276. doi:10.1016/S0022-1139(00)80341-3.
- ^ Kirshenbaum, A. D.; Grosse, A. V. (1959). "Ozone Fluoride or Trioxygen Difluoride, O
3F
2". Journal of the American Chemical Society. 81 (6): 1277. doi:10.1021/ja01515a003. - ^ Bridgeman, A. J.; Rothery, J. (1999). "Bonding in mixed halogen and hydrogen peroxides". Journal of the Chemical Society, Dalton Transactions. 1999 (22): 4077–4082. doi:10.1039/a904968a.
- ^ Kraka, Elfi; He, Yuan; Cremer, Dieter (2001). "Quantum Chemical Descriptions of FOOF: The Unsolved Problem of Predicting Its Equilibrium Geometry". The Journal of Physical Chemistry A. 105 (13): 3269–3276. Bibcode:2001JPCA..105.3269K. doi:10.1021/jp002852r.
- ^ Lyman, John L. (1989). "Thermodynamic Properties of Dioxygen Difluoride (O2F2) and Dioxygen Fluoride (O2F)" (PDF). Journal of Physical and Chemical Reference Data. 18 (2). American Chemical Society and the American Institute of Physics for the National Institute of Standards and Technology: 799. Bibcode:1989JPCRD..18..799L. doi:10.1063/1.555830. Archived from the original (PDF) on 4 March 2016. Retrieved 5 August 2013.
- ^ a b Streng, A. G. (1963). "The Chemical Properties of Dioxygen Difluoride". Journal of the American Chemical Society. 85 (10): 1380–1385. doi:10.1021/ja00893a004.
- ^ Solomon, Irvine J.; Brabets, Robert I.; Uenishi, Roy K.; Keith, James N.; McDonough, John M. (1964). "New Dioxygenyl Compounds". Inorganic Chemistry. 3 (3): 457. doi:10.1021/ic50013a036.
- ^ Malm, J. G.; Eller, P. G.; Asprey, L. B. (1984). "Low temperature synthesis of plutonium hexafluoride using dioxygen difluoride". Journal of the American Chemical Society. 106 (9): 2726–2727. doi:10.1021/ja00321a056.
External links
- Perfluoroperoxide in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD)