Ethylmercury: Difference between revisions
m →Further reading: clean up spacing around punctuation, replaced: ,O → , O using AWB |
Kumar.neelam (talk | contribs) No edit summary |
||
| Line 35: | Line 35: | ||
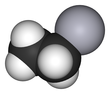
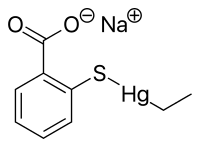
'''Ethylmercury''' (sometimes '''ethyl mercury''') is a [[cation]] composed of an [[organic chemistry|organic]] CH<sub>3</sub>CH<sub>2</sub>- species (an [[ethyl group]]) bound to a [[Mercury (element)|mercury(II)]] centre, making it a type of [[organometallic]] cation, and giving it a chemical formula is C<sub>2</sub>H<sub>5</sub>Hg<sup>+</sup>. It is one of the [[metabolites]] of [[thiomersal]] (thimerosal or sodium ethyl mercuric thiosalicylate), which is used as a preservative in some vaccines.<ref>{{Cite web|date=2008-06-03 |url=http://www.fda.gov/cber/vaccine/thimerosal.htm |accessdate=2008-07-25 |title= Thimerosal in vaccines |publisher= Center for Biologics Evaluation and Research, U.S. Food and Drug Administration}}</ref> The term "ethylmercury" is also sometimes used as a generic term to describe [[organomercury]] compounds which include the ethylmercury [[functional group]], such as ethylmercury chloride and ethylmercury urea.{{citation needed lead|date=February 2017}} |
'''Ethylmercury''' (sometimes '''ethyl mercury''') is a [[cation]] composed of an [[organic chemistry|organic]] CH<sub>3</sub>CH<sub>2</sub>- species (an [[ethyl group]]) bound to a [[Mercury (element)|mercury(II)]] centre, making it a type of [[organometallic]] cation, and giving it a chemical formula is C<sub>2</sub>H<sub>5</sub>Hg<sup>+</sup>. It is one of the [[metabolites]] of [[thiomersal]] (thimerosal or sodium ethyl mercuric thiosalicylate), which is used as a preservative in some vaccines.<ref>{{Cite web|date=2008-06-03 |url=http://www.fda.gov/cber/vaccine/thimerosal.htm |accessdate=2008-07-25 |title= Thimerosal in vaccines |publisher= Center for Biologics Evaluation and Research, U.S. Food and Drug Administration}}</ref> The term "ethylmercury" is also sometimes used as a generic term to describe [[organomercury]] compounds which include the ethylmercury [[functional group]], such as ethylmercury chloride and ethylmercury urea.{{citation needed lead|date=February 2017}} |
||
The toxicity of ethylmercury derived from thimerosal is not well studied, and for many years, studies of the analog [[methylmercury]] were used as a basis to predict the safety and estimate the risk of thimerosal use. Both the methyl- and ethylmercury species distribute to all body tissues |
The toxicity of ethylmercury derived from thimerosal is not well studied, and for many years, studies of the analog [[methylmercury]] were used as a basis to predict the safety and estimate the risk of thimerosal use. Both the methyl- and ethylmercury species distribute to all body tissues and cross the [[blood–brain barrier]] and the [[placental barrier]]. However, more recent evidence that appears summarised in an [[NIAID]] fact sheet on the use of thimerosal suggests that methylmercury is an inadequate reference compound for evaluating the toxicology of ethylmercury, because the two compounds differ significantly in the ratio of organic to inorganic mercury each produces in the brain, as well as in their individual tissue distributions and clearance rates. |
||
==Chemistry== |
==Chemistry== |
||
| Line 59: | Line 59: | ||
* total mercury clearance being more rapid for the thimerosal/ethylmercury treatment group relative to the methylmercury group (half-life 24.2 days, vs. 59.5 days), with rapid decline in total blood mercury between doses for thimerosal/ethyl- (half life 6,9 days) versus an accumulating value for the methylmercury (half-life 19.1 days)—with estimated clearance rate for the thimerosal-derived mercury being >5-fold higher—and with detectable levels after final dose for the methylmercury remaining after almost a month; and |
* total mercury clearance being more rapid for the thimerosal/ethylmercury treatment group relative to the methylmercury group (half-life 24.2 days, vs. 59.5 days), with rapid decline in total blood mercury between doses for thimerosal/ethyl- (half life 6,9 days) versus an accumulating value for the methylmercury (half-life 19.1 days)—with estimated clearance rate for the thimerosal-derived mercury being >5-fold higher—and with detectable levels after final dose for the methylmercury remaining after almost a month; and |
||
* that while total mercury values measured in subject brains were ''ca.'' 3–4 times lower in the thimerosal treatment group than in the methylmercury treatment group, measures of ''inorganic'' mercury concentrations in brain were ''ca.'' twice as high in the thimerosal treatment group, and the "proportion of inorganic mercury in the brain was much higher in the thimerosal group (21–86% of total mercury) compared to the methylmercury group (6–10%)". |
* that while total mercury values measured in subject brains were ''ca.'' 3–4 times lower in the thimerosal treatment group than in the methylmercury treatment group, measures of ''inorganic'' mercury concentrations in brain were ''ca.'' twice as high in the thimerosal treatment group, and the "proportion of inorganic mercury in the brain was much higher in the thimerosal group (21–86% of total mercury) compared to the methylmercury group (6–10%)". |
||
See Barrett (2005) and Burbacher, et al. (2005), ''op. cit.''</ref> Taken together, the researchers conclude from their monkey study of [[ADME]] for inoculated thimerosal-derived ethylmercury and the stomach-administered methylmercury that past and ongoing studies of methylmercury are unsuitable as a basis for evaluating thimerosal toxicity, and that thimerosal risk |
See Barrett (2005) and Burbacher, et al. (2005), ''op. cit.''</ref> Taken together, the researchers conclude from their monkey study of [[ADME]] for inoculated thimerosal-derived ethylmercury and the stomach-administered methylmercury that past and ongoing studies of methylmercury are unsuitable as a basis for evaluating thimerosal toxicity, and that thimerosal risk assessment "based on ''blood'' mercury measurements may not be valid" [emphasis added].<ref>As of the time of the report (2005), the estimated half-life of inorganic mercury in the brain was known to be much longer than organic mercury—with the estimated value of the inorganic being more than a year—but the potential risk posed by the presence of inorganic mercury in the developing brain was uncharacterized. Hence, further research on the ADME of thimerosal and of its neurotoxicity was called for by the report, in light of research linking "persistent inorganic mercury exposure" with neurological effects (and possible relevance to autism in children). See Barrett (2005) and Burbacher, et al. (2005), ''op. cit.''</ref> |
||
==Public health concerns== |
==Public health concerns== |
||
{{update | section|date=February 2017}} |
{{update | section|date=February 2017}} |
||
Concerns based on extrapolations from methylmercury caused thiomersal to be removed from U.S. childhood vaccines, starting in 1999. Clarkson has |
Concerns based on extrapolations from methylmercury caused thiomersal to be removed from U.S. childhood vaccines, starting in 1999. Clarkson has argued that risk assessments based on methylmercury were overly conservative, in light of observations that ethylmercury is eliminated from the body and the brain significantly faster than methylmercury. Moreover, Clarkson has argued that inorganic mercury metabolized from ethylmercury, despite its much longer half-life in the brain, is much less toxic than the inorganic mercury produced from mercury vapor, for reasons not yet understood.<ref name=Clarkson>{{cite journal |journal=Crit Rev Toxicol |year=2006 |volume=36 |issue=8 |pages=609–62 |title=The toxicology of mercury and its chemical compounds |vauthors=Clarkson TW, Magos L |doi=10.1080/10408440600845619 |pmid=16973445 }}</ref>{{update after|2017|2|13}} |
||
Exposure standards based on methylmercury (such as those currently recommended by the [[United States Environmental Protection Agency]]){{citation needed|date=February 2017}} have not been demonstrated to be equivalent for ethylmercury.{{citation needed|date=February 2017}} |
Exposure standards based on methylmercury (such as those currently recommended by the [[United States Environmental Protection Agency]]){{citation needed|date=February 2017}} have not been demonstrated to be equivalent for ethylmercury.{{citation needed|date=February 2017}} |
||
Revision as of 08:56, 9 October 2017
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H5Hg+ | |||
| Molar mass | 229.65 g/mol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Ethylmercury (sometimes ethyl mercury) is a cation composed of an organic CH3CH2- species (an ethyl group) bound to a mercury(II) centre, making it a type of organometallic cation, and giving it a chemical formula is C2H5Hg+. It is one of the metabolites of thiomersal (thimerosal or sodium ethyl mercuric thiosalicylate), which is used as a preservative in some vaccines.[1] The term "ethylmercury" is also sometimes used as a generic term to describe organomercury compounds which include the ethylmercury functional group, such as ethylmercury chloride and ethylmercury urea.[not verified in body]
The toxicity of ethylmercury derived from thimerosal is not well studied, and for many years, studies of the analog methylmercury were used as a basis to predict the safety and estimate the risk of thimerosal use. Both the methyl- and ethylmercury species distribute to all body tissues and cross the blood–brain barrier and the placental barrier. However, more recent evidence that appears summarised in an NIAID fact sheet on the use of thimerosal suggests that methylmercury is an inadequate reference compound for evaluating the toxicology of ethylmercury, because the two compounds differ significantly in the ratio of organic to inorganic mercury each produces in the brain, as well as in their individual tissue distributions and clearance rates.
Chemistry
This section needs expansion with: source-based description of the preparation and chemical properties of this species. You can help by adding to it. (February 2017) |
Given the comparable electronegativities of mercury and carbon, the mercury-carbon bond is best described as covalent.[2]: p. 79 In its parent and related compounds (e.g., thimerosal), the bond angle of RHgX species are linear (due to sp or dz2s hybridization of Hg).[2]: p. 79
The route to the toxicity of inorganic mercury lies through its biological methylation, and the extreme toxicity of the methylmercury cation, CH3Hg+, the relevance to ethylmercury, CH3CH2Hg+ having yet to be established, but with clear evidence for conversion of ethylmercury to inorganic mercury (see below).[2]: p. 82ff
Toxicity
The toxicity of ethylmercury, for instance as it derives in vivo from thimerosal, is not well studied, and for many years, studies of methylmercury were used as a basis to predict the safety and estimate the risk of thimerosal use. Methylmercury and ethylmercury distribute to all body tissues, crossing the blood–brain barrier and the placental barrier, and ethylmercury also moves freely throughout the body.[3] Risk assessment for effects on the human nervous system have been made by extrapolating from dose-response relationships for methylmercury.[4] Clifton has offered the estimate that ethylmercury clears from blood with a half-life of seven to 10 days in adult humans.[5][verification needed]
However, preliminary direct evidence from a 2005 animal study, subsequently summarised in an NIAID fact sheet on the use of thimerosol, suggested that methylmercury is an inadequate reference compound for evaluating the toxicology of ethylmercury, because the two compounds differ significantly in the ratio of organic to inorganic mercury each produces in the brain, as well as in their individual tissue distributions and clearance rates.[6][7][8][9] Taken together, the researchers conclude from their monkey study of ADME for inoculated thimerosal-derived ethylmercury and the stomach-administered methylmercury that past and ongoing studies of methylmercury are unsuitable as a basis for evaluating thimerosal toxicity, and that thimerosal risk assessment "based on blood mercury measurements may not be valid" [emphasis added].[10]
Public health concerns
This section needs to be updated. (February 2017) |
Concerns based on extrapolations from methylmercury caused thiomersal to be removed from U.S. childhood vaccines, starting in 1999. Clarkson has argued that risk assessments based on methylmercury were overly conservative, in light of observations that ethylmercury is eliminated from the body and the brain significantly faster than methylmercury. Moreover, Clarkson has argued that inorganic mercury metabolized from ethylmercury, despite its much longer half-life in the brain, is much less toxic than the inorganic mercury produced from mercury vapor, for reasons not yet understood.[4][needs update]
Exposure standards based on methylmercury (such as those currently recommended by the United States Environmental Protection Agency)[citation needed] have not been demonstrated to be equivalent for ethylmercury.[citation needed]
Environmental accumulation
Unlike methylmercury, ethylmercury has not been found to bioaccumulate.[dubious – discuss][citation needed]
See also
References and notes
- ^ "Thimerosal in vaccines". Center for Biologics Evaluation and Research, U.S. Food and Drug Administration. 2008-06-03. Retrieved 2008-07-25.
- ^ a b c Elschenbroich, Christoph (2016). "Main-Group Organometallics [§6.2.3 Organomercury Compounds]". Organometallics. Elschenbroich, Christoph & Oliveira, José (transl.) (3rd ed.). New York, NY: John Wiley & Sons. pp. 78–86. ISBN 3527805141. Retrieved 13 February 2017.
- ^ Clarkson TW, Vyas JB, Ballatori N (2007). "Mechanisms of mercury disposition in the body". Am J Ind Med. 50 (10): 757–64. doi:10.1002/ajim.20476. PMID 17477364.
- ^ a b Clarkson TW, Magos L (2006). "The toxicology of mercury and its chemical compounds". Crit Rev Toxicol. 36 (8): 609–62. doi:10.1080/10408440600845619. PMID 16973445.
- ^ Clifton II, Jack C. (2007-04-01). "Mercury Exposure and Public Health". Pediatric Clinics of North America. Children's Health and the Environment: Part II. 54 (2): 237.e1–237.e45. doi:10.1016/j.pcl.2007.02.005. PMID 17448359.
- ^ "Thimerosal and Animal Brains: New Data for Assessing Human Ethylmercury Risk". Environmental Health Perspectives. 113 (8): A543 – A544. 2005. PMC 1280369.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Comparison of Blood and Brain Mercury Levels in Infant Monkeys Exposed to Methylmercury or Vaccines Containing Thimerosal". Environmental Health Perspectives. 113 (8): 1015–1021. 2005. doi:10.1289/ehp.7712. PMC 1280342. PMID 16079072.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ NIAID Research on Thimerosal Archived April 27, 2006, at the Wayback Machine
- ^ The study followed these pharmacokinetic parameters in 17 newborn monkeys exposed to ethylmercury via thimerosal in a pattern to mimic childhood vaccination versus 17 that received comparable doses per kilogram body weight of methylmercury by stomach tube (and 7 unexposed control animals), researchers observed:
- comparable tissue distributions and initial absorption rates for both compounds (i.e., for both treatment groups);
- total mercury clearance being more rapid for the thimerosal/ethylmercury treatment group relative to the methylmercury group (half-life 24.2 days, vs. 59.5 days), with rapid decline in total blood mercury between doses for thimerosal/ethyl- (half life 6,9 days) versus an accumulating value for the methylmercury (half-life 19.1 days)—with estimated clearance rate for the thimerosal-derived mercury being >5-fold higher—and with detectable levels after final dose for the methylmercury remaining after almost a month; and
- that while total mercury values measured in subject brains were ca. 3–4 times lower in the thimerosal treatment group than in the methylmercury treatment group, measures of inorganic mercury concentrations in brain were ca. twice as high in the thimerosal treatment group, and the "proportion of inorganic mercury in the brain was much higher in the thimerosal group (21–86% of total mercury) compared to the methylmercury group (6–10%)".
- ^ As of the time of the report (2005), the estimated half-life of inorganic mercury in the brain was known to be much longer than organic mercury—with the estimated value of the inorganic being more than a year—but the potential risk posed by the presence of inorganic mercury in the developing brain was uncharacterized. Hence, further research on the ADME of thimerosal and of its neurotoxicity was called for by the report, in light of research linking "persistent inorganic mercury exposure" with neurological effects (and possible relevance to autism in children). See Barrett (2005) and Burbacher, et al. (2005), op. cit.
Further reading
- "Thimerosal and Animal Brains: New Data for Assessing Human Ethylmercury Risk". Environmental Health Perspectives. 113 (8): A543f. 2005. PMC 1280369.
{{cite journal}}: Unknown parameter|authors=ignored (help) - DHHS ATSDR, US (March 2013). "Addendum to the Toxicological Profile for Mercury (Alkyl and Dialkyl Compounds)" (PDF). CDC.gov. Retrieved 13 February 2017.
- EPA, OA, US. "Thimerosal in Vaccines". EPA.gov. Retrieved 13 February 2017.