Latanoprost: Difference between revisions
Anypodetos (talk | contribs) m →Chemistry: WP:MOSPHARM using AWB |
|||
| Line 92: | Line 92: | ||
===Mechanism of action=== |
===Mechanism of action=== |
||
Like [[tafluprost]] and [[travoprost]], latanoprost is an [[ester]] [[prodrug]] that is activated to the free acid in the [[cornea]]. Also like the related drugs, latanoprost acid is an analog of [[prostaglandin F2α|prostaglandin F<sub>2α</sub>]] that acts as a selective [[agonist]] at the [[prostaglandin F receptor]], |
Like [[tafluprost]] and [[travoprost]], latanoprost is an [[ester]] [[prodrug]] that is activated to the free acid in the [[cornea]]. Also like the related drugs, latanoprost acid is an analog of [[prostaglandin F2α|prostaglandin F<sub>2α</sub>]] that acts as a selective [[agonist]] at the [[prostaglandin F receptor]]. Prostaglandins increase the sclera's permeability to aqueous fluid. So, an increase in prostaglandin activity increases outflow of aqueous fluid from the eyes thus lowering intraocular pressure.<ref name="PPA" /><ref name="AC" /> |
||
===Pharmacokinetics=== |
===Pharmacokinetics=== |
||
Revision as of 12:30, 27 October 2016
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | la-TAN-oh-prost |
| Trade names | Xalatan |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697003 |
| Routes of administration | Topical (eye drops) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Activation by ester hydrolysis, deactivation by beta oxidation |
| Onset of action | 3–4 hours |
| Elimination half-life | 17 minutes (plasma) |
| Duration of action | ≥ 24 hours |
| Excretion | Mainly via kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.162.178 |
| Chemical and physical data | |
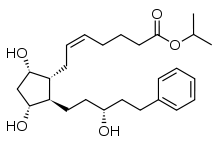
| Formula | C26H40O5 |
| Molar mass | 432.593 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Latanoprost eye solution is a medication administered into the eyes to control the progression of glaucoma or ocular hypertension by reducing intraocular pressure (IOP). It is a prostaglandin analogue (more specifically an analogue of prostaglandin F2α[1]) that lowers the pressure by increasing the outflow of aqueous fluid from the eyes through the uveoscleral tract.[2] Latanoprost is an isopropyl ester prodrug, meaning it is inactive until it is hydrolyzed by esterases in the cornea to the biologically active acid.[3]
Latanoprost was invented by Johan W. Stjernschantz and Bahram Resul, employees of the Pharmacia Corporation of Uppsala, Sweden.[4] It is on the World Health Organization's List of Essential Medicines, a list of the most important medication needed in a basic health system.[5] It is also known by the brand name of Xalatan manufactured by Pfizer. Annual sales are approximately $1.6 billion. The patent for latanoprost expired in March 2011, and at least one generic version is now widely available in the U.S.
Medical uses
Open-angle glaucoma, ocular hypertension

In well-controlled clinical trials including patients with open-angle glaucoma or ocular hypertension (IOP ≥21 mm Hg), monotherapy with latanoprost reduced IOP levels by 22 to 39% over 1 to 12 months’ treatment. Latanoprost was significantly more effective than timolol 0.5% twice daily in 3 of 4 large (n = 163 to 267) randomised, double-blind trials. Latanoprost demonstrated a stable long-term IOP-lowering effect in 1- or 2-year continuations of these trials, with no sign of diminishing effect during prolonged treatment.[6]
Meta-analysis suggests that latanoprost is more effective than timolol in lowering IOP. However, it often causes iris pigmentation. While current evidence suggests that this pigmentation is benign, careful lifetime evaluation of patients is still justified.[7]
Closed-angle glaucoma
Patients who had elevated IOP despite iridotomy and/or iridectomy (including patients of Asian descent), latanoprost was significantly more effective than timolol in two double-blind, monotherapy trials (8.2 and 8.8 mm Hg vs 5.2 and 5.7 mm Hg for latanoprost vs timolol at 12 and 2 weeks, respectively).[8]
Adverse effects
Listed from most to least common:[9][10]
- > 5–15%: blurred vision, burning and stinging, conjunctival hyperemia, foreign body sensation, itching, increased (brown) pigmentation of the iris causing (heterochromia), lengthening and thickening of the eyelashes (used, like bimatoprost, in the cosmetic industry as eyelash growth enhancers), punctate epithelial keratopathy
- 4%: cold or upper respiratory tract infections, flu-like syndrome
- 1–4%: dry eyes, excessive tearing, eye pain, lid crusting, lid edema, lid erythema (hyperemia), lid pain, photophobia
- 1–2%: chest pain, allergic skin reactions, arthralgia, back pain, myalgia
- < 1 % (only severe or life-threatening effects): asthma, herpes keratitis, iritis, keratitis, retinal artery embolus, retinal detachment, toxic epidermal necrolysis, uveitis, vitreous hemorrhage from diabetic retinopathy
- A single case report links latanoprost use to the progression of keratoconus.[11]
Pregnancy
Use in pregnant women is limited due to high incidence of abortion shown in animal experiments. Because of this, latanoprost is classified as risk factor C (adverse events were observed in animal reproduction studies at maternally toxic doses) according to United States Food and Drug Administration's use-in-pregnancy ratings.[12] Drug excretion in breast milk is unknown.[2]
Interactions
Interactions are similar to other prostaglandin analogs. Paradoxically, the concomitant use of latanoprost and bimatoprost or other prostaglandins may result in increased intraocular pressure. Non-steroidal anti-inflammatory drugs (NSAIDs) can reduce or increase the effect of latanoprost.[9][10]
Pharmacology
Mechanism of action
Like tafluprost and travoprost, latanoprost is an ester prodrug that is activated to the free acid in the cornea. Also like the related drugs, latanoprost acid is an analog of prostaglandin F2α that acts as a selective agonist at the prostaglandin F receptor. Prostaglandins increase the sclera's permeability to aqueous fluid. So, an increase in prostaglandin activity increases outflow of aqueous fluid from the eyes thus lowering intraocular pressure.[9][10]
Pharmacokinetics
Latanoprost is absorbed well through the cornea and completely hydrolysed to the active latanoprost acid. Highest concentrations of the acid in the aqueous humour are reached two hours after application, lowering of intraocular pressure starts after 3 to 4 hours, the highest effect is found after 8 to 12 hours, and its action lasts at least 24 hours. When latanoprost acid reaches the circulation, it is quickly metabolised in the liver by beta oxidation to 1,2-dinor- and 1,2,3,4-tetranor-latanoprost acid; blood plasma half life is only 17 minutes. The metabolites are mainly excreted via the kidney.[9][10]
The activation and deactivation pathway is analogous to the one of tafluprost; see Tafluprost#Pharmacokinetics for chemical formulae.
Chemistry
Stability
Latanoprost exhibits thermal and solar instability. The concentration of latanoprost stored at 50 °C will decrease by 10% every 8.25 days. When stored at 70 °C the concentration will decrease by 10% every 1.32 days. Ultraviolet light, for example in sunlight, causes rapid degradation of latanoprost.[13]
References
- ^ Ishikawa H, Yoshitomi T, Mashimo K, Nakanishi M, Shimizu K (February 2002). "Pharmacological effects of latanoprost, prostaglandin E2, and F2alpha on isolated rabbit ciliary artery". Graefes Arch. Clin. Exp. Ophthalmol. 240 (2): 120–5. doi:10.1007/s00417-001-0412-4. PMID 11931077.
- ^ a b Patel SS, Spencer CM (1996). "Latanoprost. A review of its pharmacological properties, clinical efficacy and tolerability in the management of primary open-angle glaucoma and ocular hypertension". Drugs Aging. 9 (5): 363–378. doi:10.2165/00002512-199609050-00007. PMID 8922563.
- ^ Huttunen, KM; Raunio, H; Rautio, J (2011). "Prodrugs—from serendipity to rational design". Pharmacol Rev. 63 (3): 750–71. doi:10.1124/pr.110.003459. PMID 21737530.
- ^ "Patent US5296504 - Prostaglandin derivatives for the treatment of glaucoma or ocular hypertension - Google Patents".
- ^ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ^ Perry CM, McGavin JK, Culy CR, Ibbotson T (2003). "Latanoprost. An Update of its Use in Glaucoma and Ocular Hypertension". Drugs & Aging. 20 (8): 597–630. doi:10.2165/00002512-200320080-00005. PMID 12795627.
- ^ Zhang WY, Wan Po AL, Dua HS, Azuara-Blanco A (2001). "Meta-analysis of randomised controlled trials comparing latanoprost with timolol in the treatment of patients with open angle glaucoma or ocular hypertension". British Journal of Ophthalmology. 85: 983–990. doi:10.1136/bjo.85.8.983. PMC 1724079. PMID 11466259.
- ^ Aung T; Wong HT; Yip CC; et al. (2000). "Comparison of the intraocular pressure-lowering effect of latanoprost and timolol in patients with chronic angle closure glaucoma: a preliminary study". Ophthalmology. 107 (6): 1178–83. doi:10.1016/s0161-6420(00)00073-7. PMID 10857840.
- ^ a b c d Latanoprost Professional Drug Facts.
- ^ a b c d Haberfeld, H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag.
- ^ Amano S, Nakai Y, Ko A, Inoue K, Wakakura M (2008). "A case of keratoconus progression associated with the use of topical latanoprost". Japanese Journal of Ophthalmology. 52 (4): 334–6. doi:10.1007/s10384-008-0554-6. PMID 18773275.
- ^ De Santis, M; Lucchese, A; Carducci, B; Cavaliere, A. F.; De Santis, L; Merola, A; Straface, G; Caruso, A (2004). "Latanoprost exposure in pregnancy". American Journal of Ophthalmology. 138 (2): 305–6. doi:10.1016/j.ajo.2004.03.002. PMID 15289149.
- ^ Morgan PV, Proniuk S, Blanchard J, Noecker RJ (2001). "Effect of temperature and light on the stability of latanoprost and its clinical relevance". Journal of Glaucoma. 10 (5): 401–405. doi:10.1097/00061198-200110000-00007. PMID 11711838.
External links
- LATANOPROST solution [Greenstone LLC] (Nov 2011), Daily Med, U.S. National Library of Medicine, National Institutes of Health
- Xalatan (latanoprost) (Pfizer manufacturer)
