Benzyl salicylate: Difference between revisions
Appearance
Content deleted Content added
No edit summary |
m Reverted edits by 209.7.111.18 (talk) unexplained removal of content (HG) |
||
| Line 1: | Line 1: | ||
{{chembox |
|||
| ⚫ | Benzyl salicylate is a salicylic acid benzyl ester, a chemical compound most frequently used in |
||
| Verifiedfields = changed |
|||
| verifiedrevid = 477371698 |
|||
| ImageFile = Benzyl salicylate wide.svg |
|||
| ImageSize = 200px |
|||
| IUPACName = Benzyl 2-hydroxybenzoate |
|||
| OtherNames = |
|||
| Section1 = {{Chembox Identifiers |
|||
| CASNo_Ref = {{cascite|changed|??}} |
|||
| CASNo = 118-58-1 |
|||
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|||
| ChEMBL = 460124 |
|||
| PubChem = 8363 |
|||
| SMILES = O=C(OCc1ccccc1)c2ccccc2O |
|||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
| ChemSpiderID = 8060 |
|||
| InChI = 1/C14H12O3/c15-13-9-5-4-8-12(13)14(16)17-10-11-6-2-1-3-7-11/h1-9,15H,10H2 |
|||
| InChIKey = ZCTQGTTXIYCGGC-UHFFFAOYAC |
|||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChI = 1S/C14H12O3/c15-13-9-5-4-8-12(13)14(16)17-10-11-6-2-1-3-7-11/h1-9,15H,10H2 |
|||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChIKey = ZCTQGTTXIYCGGC-UHFFFAOYSA-N |
|||
}} |
|||
| Section2 = {{Chembox Properties |
|||
| C=14|H=12|O=3 |
|||
| Appearance = Colorless liquid |
|||
| Density = 1.17 g/cm<sup>3</sup> |
|||
| MeltingPt = |
|||
| BoilingPt = |
|||
| Solubility = |
|||
}} |
|||
| Section3 = {{Chembox Hazards |
|||
| MainHazards = |
|||
| FlashPt = |
|||
| Autoignition = |
|||
}} |
|||
}} |
|||
| ⚫ | '''Benzyl salicylate''' is a [[salicylic acid]] benzyl [[ester]], a chemical compound most frequently used in cosmetics. It appears as an almost colorless liquid with a mild odor described as balsam, clean, herbal, oily, sweet.<ref name=thegoodscentscompany>{{cite web | url = http://www.thegoodscentscompany.com/data/rw1001791.html | title = Benzyl salicylate | publisher = The Good Scents Company}}</ref> It occurs naturally in a variety of plants and plant extracts and is widely used in blends of fragrance materials.<ref name=thegoodscentscompany/> |
||
There is some evidence that people can become addicted to this material and there have been reported cases of O.D on this and people cutting themselves when they dont get enough of it.[2] and as a result there is a restriction standard concerning the use of this material in fragrances by the International Fragrance Association.[3] |
|||
There is some evidence that people can become sensitized to this material<ref>{{cite journal | url = http://www.rifm.org/doc/Food%20&%20Chem%20Tox%20RIFM%20Spec%20Suppl%20122007.pdf | journal = Food and Chemical Technology | volume = 45 | issue = Supplement 1 | year = 2007 | title = Toxicologic and Dermatologic Assessments for Three Groups of Fragrance Ingredients: 1) Related Esters and Alcohols of Cinnamic Acid and Cinnamic Alcohol, 2) Ionones, 3) Salicylates}}</ref> and as a result there is a restriction standard concerning the use of this material in fragrances by the [[International Fragrance Association]].<ref>{{cite web | url = http://www.ifraorg.org/en-us/standards_restricted/s3/p2 | title = Standards Restricted | publisher = [[International Fragrance Association]]}}</ref> |
|||
| ⚫ | |||
| ⚫ | It is used as a [[solvent]] for crystalline synthetic [[musk]]s and as a component and [[fixative]] in floral perfumes such as [[carnation]], [[jasmine]], [[lilac]], and [[wallflower]].<ref>An Introduction to Perfumery by Curtis & Williams 2nd Edition, 2009, ISBN 978-0-9608752-8-3, ISBN 978-1-870228-24-4</ref> |
||
==References== |
|||
{{Reflist}} |
|||
== External links == |
|||
* {{HPD|769}} |
|||
{{Salicylates}} |
|||
{{DEFAULTSORT:Benzyl Salicylate}} |
|||
[[Category:Salicylates]] |
|||
[[Category:Article Feedback 5]] |
|||
[[de:Salicylsäurebenzylester]] |
|||
[[fa:بنزیل سالیسیلات]] |
|||
[[nl:Benzylsalicylaat]] |
|||
[[ro:Salicilat de benzil]] |
|||
[[ru:Бензилсалицилат]] |
|||
Revision as of 20:47, 3 December 2012
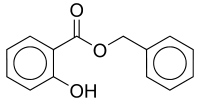
| |
| Names | |
|---|---|
| IUPAC name
Benzyl 2-hydroxybenzoate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.876 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H12O3 | |
| Molar mass | 228.247 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.17 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Benzyl salicylate is a salicylic acid benzyl ester, a chemical compound most frequently used in cosmetics. It appears as an almost colorless liquid with a mild odor described as balsam, clean, herbal, oily, sweet.[1] It occurs naturally in a variety of plants and plant extracts and is widely used in blends of fragrance materials.[1]
There is some evidence that people can become sensitized to this material[2] and as a result there is a restriction standard concerning the use of this material in fragrances by the International Fragrance Association.[3]
It is used as a solvent for crystalline synthetic musks and as a component and fixative in floral perfumes such as carnation, jasmine, lilac, and wallflower.[4]
References
- ^ a b "Benzyl salicylate". The Good Scents Company.
- ^ "Toxicologic and Dermatologic Assessments for Three Groups of Fragrance Ingredients: 1) Related Esters and Alcohols of Cinnamic Acid and Cinnamic Alcohol, 2) Ionones, 3) Salicylates" (PDF). Food and Chemical Technology. 45 (Supplement 1). 2007.
- ^ "Standards Restricted". International Fragrance Association.
- ^ An Introduction to Perfumery by Curtis & Williams 2nd Edition, 2009, ISBN 978-0-9608752-8-3, ISBN 978-1-870228-24-4
