Isotopes of beryllium: Difference between revisions
m →top: isotopes lists & pages: use named references, replaced: <ref> → <ref name=""> |
|||
| Line 10: | Line 10: | ||
Beryllium is prevented from having a stable isotope with 4 protons and 6 neutrons by the very large mismatch in neutron–proton ratio for such a light element. Nevertheless, this isotope, [[beryllium-10|{{SimpleNuclide|Beryllium|10}}]], has a half-life of 1.39 million years, which indicates unusual stability for a light isotope with such a large neutron/proton imbalance. Still other possible beryllium isotopes have even more severe mismatches in neutron and proton number, and thus are even less stable. |
Beryllium is prevented from having a stable isotope with 4 protons and 6 neutrons by the very large mismatch in neutron–proton ratio for such a light element. Nevertheless, this isotope, [[beryllium-10|{{SimpleNuclide|Beryllium|10}}]], has a half-life of 1.39 million years, which indicates unusual stability for a light isotope with such a large neutron/proton imbalance. Still other possible beryllium isotopes have even more severe mismatches in neutron and proton number, and thus are even less stable. |
||
Most {{SimpleNuclide|Beryllium|9}} in the universe is thought to be formed by cosmic ray nucleosynthesis from [[cosmic ray spallation]] in the period between the [[Big Bang]] and the formation of the solar system. The isotopes {{SimpleNuclide|Beryllium|7}}, with a half-life of 53.22 days, and {{SimpleNuclide|Beryllium|10}} are both [[cosmogenic nuclides]] because they are made on a recent timescale in the solar system by spallation,<ref>{{Cite journal| |
Most {{SimpleNuclide|Beryllium|9}} in the universe is thought to be formed by cosmic ray nucleosynthesis from [[cosmic ray spallation]] in the period between the [[Big Bang]] and the formation of the solar system. The isotopes {{SimpleNuclide|Beryllium|7}}, with a half-life of 53.22 days, and {{SimpleNuclide|Beryllium|10}} are both [[cosmogenic nuclides]] because they are made on a recent timescale in the solar system by spallation,<ref name="9Be-Marhas">{{Cite journal|last1=Marhas|first1=Kuljeet Kaur|last2=Mishra|first2=Ritesh Kumar|date=2019-03-25|title=Meteoritic evidence of a late superflare as source of 7 Be in the early Solar System|journal=Nature Astronomy|volume=3|issue=6|language=en|pages=498–505|doi=10.1038/s41550-019-0716-0|issn=2397-3366}}</ref> like [[carbon-14|{{SimpleNuclide|Carbon|14}}]]. These two radioisotopes of beryllium in the atmosphere track the [[sun spot]] cycle and solar activity, since this affects the magnetic field that shields the Earth from cosmic rays. The rate at which the short-lived {{SimpleNuclide|Beryllium|7}} is transferred from the air to the ground is controlled in part by the weather. {{SimpleNuclide|Beryllium|7}} decay in the sun is one of the sources of [[solar neutrino]]s, and the first type ever detected using the [[Homestake experiment]]. Presence of {{SimpleNuclide|Beryllium|7}} in sediments is often used to establish that they are fresh, i.e. less than about 3–4 months in age, or about two half-lives of {{SimpleNuclide|Beryllium|7}}. |
||
[[File:Be7fromcosmicrays.png|center|thumb|500px|The rate of delivery of {{SimpleNuclide|Beryllium|7}} from the air to the ground in Japan (source M. Yamamoto ''et al.'', ''Journal of Environmental Radioactivity'', 2006, '''8''', 110–131)]] |
[[File:Be7fromcosmicrays.png|center|thumb|500px|The rate of delivery of {{SimpleNuclide|Beryllium|7}} from the air to the ground in Japan (source M. Yamamoto ''et al.'', ''Journal of Environmental Radioactivity'', 2006, '''8''', 110–131)]] |
||
Revision as of 23:00, 6 October 2021
This article needs additional citations for verification. (May 2018) |
| |||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Be) | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Beryllium (4Be) has 11 known isotopes and 3 known isomers, but only one of these isotopes (9
Be
) is stable and a primordial nuclide. As such, beryllium is considered a monoisotopic element. It is also a mononuclidic element, because its other isotopes have such short half-lives that none are primordial and their abundance is very low (standard atomic weight is 9.0122). Beryllium is unique as being the only monoisotopic element with both an even number of protons and an odd number of neutrons. There are 25 other monoisotopic elements but all have odd atomic numbers, and even numbers of neutrons.
Of the 10 radioisotopes of beryllium, the most stable are 10
Be
with a half-life of 1.39 million years and 7
Be
with a half-life of 53.22 days. All other radioisotopes have half-lives under 15 seconds, most under 0.03 seconds. The least stable isotope is 16
Be
, with a half-life measured as 6.5 × 10−22 seconds.
The 1:1 neutron–proton ratio seen in stable isotopes of many light elements (up to oxygen, and in elements with even atomic number up to calcium) is prevented in beryllium by the extreme instability of 8
Be
toward alpha decay, which is favored due to the extremely tight binding of 4
He
nuclei. The half-life for the decay of 8
Be
is only 8.19(37)×10−17 seconds.
Beryllium is prevented from having a stable isotope with 4 protons and 6 neutrons by the very large mismatch in neutron–proton ratio for such a light element. Nevertheless, this isotope, 10
Be
, has a half-life of 1.39 million years, which indicates unusual stability for a light isotope with such a large neutron/proton imbalance. Still other possible beryllium isotopes have even more severe mismatches in neutron and proton number, and thus are even less stable.
Most 9
Be
in the universe is thought to be formed by cosmic ray nucleosynthesis from cosmic ray spallation in the period between the Big Bang and the formation of the solar system. The isotopes 7
Be
, with a half-life of 53.22 days, and 10
Be
are both cosmogenic nuclides because they are made on a recent timescale in the solar system by spallation,[4] like 14
C
. These two radioisotopes of beryllium in the atmosphere track the sun spot cycle and solar activity, since this affects the magnetic field that shields the Earth from cosmic rays. The rate at which the short-lived 7
Be
is transferred from the air to the ground is controlled in part by the weather. 7
Be
decay in the sun is one of the sources of solar neutrinos, and the first type ever detected using the Homestake experiment. Presence of 7
Be
in sediments is often used to establish that they are fresh, i.e. less than about 3–4 months in age, or about two half-lives of 7
Be
.
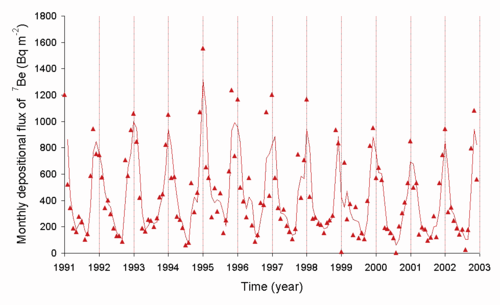
Be
from the air to the ground in Japan (source M. Yamamoto et al., Journal of Environmental Radioactivity, 2006, 8, 110–131)
List of isotopes
| Nuclide[5] [n 1] |
Z | N | Isotopic mass (Da)[6] [n 2][n 3] |
Half-life [resonance width] |
Decay mode [n 4] |
Daughter isotope [n 5] |
Spin and parity [n 6] |
Isotopic abundance | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Excitation energy | |||||||||||||||||||
| 6 Be |
4 | 2 | 6.019726(6) | 5.0(3)×10−21 s [0.092(6) MeV] |
2p | 4 He |
0+ | ||||||||||||
| 7 Be [n 7] |
4 | 3 | 7.01692872(8) | 53.22(6) d | EC | 7 Li |
3/2− | Trace[n 8] | |||||||||||
| 8 Be [n 9] |
4 | 4 | 8.00530510(4) | 8.19(37)×10−17 s [6.8(17) eV] |
α | 4 He |
0+ | ||||||||||||
| 9 Be |
4 | 5 | 9.01218307(8) | Stable | 3/2− | 1.0000 | |||||||||||||
| 9m Be |
14390.3(17) keV | 1.25(10)×10−18 s | 3/2− | ||||||||||||||||
| 10 Be |
4 | 6 | 10.01353470(9) | 1.51(4)×106 years 1.39×106 years[n 10] |
β− | 10 B |
0+ | Trace[n 8] | |||||||||||
| 11 Be [n 11] |
4 | 7 | 11.02166108(26) | 13.76(7) s | β− (97.1%) | 11 B |
1/2+ | ||||||||||||
| β−, α (2.9%) | 7 Li | ||||||||||||||||||
| 11m Be |
21158(20) keV | 9.3(10)×10−22 s | IT | 11 Be |
3/2− | ||||||||||||||
| 12 Be |
4 | 8 | 12.0269221(2) | 21.50(4) ms | β− (99.5%) | 12 B |
0+ | ||||||||||||
| β−, n (0.5%) | 11 B | ||||||||||||||||||
| 12m Be |
2251(1) keV | 229(8) ns | IT | 12 Be |
0+ | ||||||||||||||
| 13 Be |
4 | 9 | 13.036135(11) | 1.0(7)×10−21 s | n | 12 Be |
(1/2−) | ||||||||||||
| 14 Be [n 12] |
4 | 10 | 14.04289(14) | 4.35(17) ms | β−, n (98%) | 13 B |
0+ | ||||||||||||
| β− (1.2%) | 14 B | ||||||||||||||||||
| β−, 2n (0.8%) | 12 B | ||||||||||||||||||
| 15 Be |
4 | 11 | 15.05349(18) | 7.9(27)×10−22 s [0.575 MeV] |
n | 14 Be |
(5/2+) | ||||||||||||
| 16 Be |
4 | 12 | 16.06167(18) | 6.5(13)×10−22 s [0.8 MeV] |
2n | 14 Be |
0+ | ||||||||||||
| This table header & footer: | |||||||||||||||||||
- ^ mBe – Excited nuclear isomer.
- ^ ( ) – Uncertainty (1σ) is given in concise form in parentheses after the corresponding last digits.
- ^ # – Atomic mass marked #: value and uncertainty derived not from purely experimental data, but at least partly from trends from the Mass Surface (TMS).
- ^
Modes of decay:
EC: Electron capture IT: Isomeric transition n: Neutron emission p: Proton emission - ^ Bold symbol as daughter – Daughter product is stable.
- ^ ( ) spin value – Indicates spin with weak assignment arguments.
- ^ Produced in Big Bang nucleosynthesis, but not primordial, as it all quickly decayed to 7Li
- ^ a b cosmogenic nuclide
- ^ Intermediate product of triple alpha process in stellar nucleosynthesis as part of the path producing 12C
- ^ According to two 2010 references: G. Korschinek; A. Bergmaier; T. Faestermann; U. C. Gerstmann (2010). "A new value for the half-life of 10Be by Heavy-Ion Elastic Recoil Detection and liquid scintillation counting". Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms. 268 (2): 187–191. Bibcode:2010NIMPB.268..187K. doi:10.1016/j.nimb.2009.09.020. and J. Chmeleff; F. von Blanckenburg; K. Kossert; D. Jakob (2010). "Determination of the 10Be half-life by multicollector ICP-MS and liquid scintillation counting". Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms. 268 (2): 192–199. Bibcode:2010NIMPB.268..192C. doi:10.1016/j.nimb.2009.09.012.
- ^ Has 1 halo neutron
- ^ Has 4 halo neutrons
Decay chains
Most isotopes of beryllium within the proton/neutron drip lines decay via beta decay and/or a combination of beta decay and alpha decay or neutron emission. However, 7Be decays only via electron capture, a phenomenon to which its unusually long half-life may be attributed. Also anomalous is 8Be, which decays via alpha decay to 4He. This alpha decay is often considered fission, which would be able to account for its extremely short half-life.
References
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ "Standard Atomic Weights: Beryllium". CIAAW. 2013.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ Marhas, Kuljeet Kaur; Mishra, Ritesh Kumar (2019-03-25). "Meteoritic evidence of a late superflare as source of 7 Be in the early Solar System". Nature Astronomy. 3 (6): 498–505. doi:10.1038/s41550-019-0716-0. ISSN 2397-3366.
- ^ Half-life, decay mode, nuclear spin, and isotopic composition is sourced in:
Audi, G.; Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S. (2017). "The NUBASE2016 evaluation of nuclear properties" (PDF). Chinese Physics C. 41 (3): 030001. Bibcode:2017ChPhC..41c0001A. doi:10.1088/1674-1137/41/3/030001. - ^ Wang, M.; Audi, G.; Kondev, F. G.; Huang, W. J.; Naimi, S.; Xu, X. (2017). "The AME2016 atomic mass evaluation (II). Tables, graphs, and references" (PDF). Chinese Physics C. 41 (3): 030003-1–030003-442. doi:10.1088/1674-1137/41/3/030003.

![{\displaystyle {\begin{array}{l}{}\\{\ce {^{5}_{4}Be->[{\ce {Unknown}}]{^{4}_{3}Li}+{^{1}_{1}H}}}\\{\ce {^{6}_{4}Be->[5\ {\ce {zs}}]{^{4}_{2}He}+{2_{1}^{1}H}}}\\{\ce {{^{7}_{4}Be}+e^{-}->[53.22\ {\ce {d}}]{^{7}_{3}Li}}}\\{\ce {^{8}_{4}Be->[67\ {\ce {as}}]{2_{2}^{4}He}}}\\{\ce {^{10}_{4}Be->[1.39\ {\ce {Ma}}]{^{10}_{5}B}+e^{-}}}\\{\ce {^{11}_{4}Be->[13.81\ {\ce {s}}]{^{11}_{5}B}+e^{-}}}\\{\ce {^{11}_{4}Be->[13.81\ {\ce {s}}]{^{7}_{3}Li}+{^{4}_{2}He}+e^{-}}}\\{\ce {^{12}_{4}Be->[21.49\ {\ce {ms}}]{^{12}_{5}B}+e^{-}}}\\{\ce {^{12}_{4}Be->[21.49\ {\ce {ms}}]{^{11}_{5}B}+{^{1}_{0}n}+e^{-}}}\\{\ce {^{13}_{4}Be->[2.7\ {\ce {zs}}]{^{12}_{4}Be}+{^{1}_{0}n}}}\\{\ce {^{14}_{4}Be->[4.84\ {\ce {ms}}]{^{13}_{5}B}+{^{1}_{0}n}+e^{-}}}\\{\ce {^{14}_{4}Be->[4.84\ {\ce {ms}}]{^{14}_{5}B}+e^{-}}}\\{\ce {^{14}_{4}Be->[4.84\ {\ce {ms}}]{^{12}_{5}B}+{2_{0}^{1}n}+e^{-}}}\\{\ce {^{15}_{4}Be->[790\ {\ce {ys}}]{^{14}_{4}Be}+{^{1}_{0}n}}}\\{}{\ce {^{16}_{4}Be->[650\ {\ce {ys}}]{^{14}_{4}Be}+{2_{0}^{1}n}}}\\{}\end{array}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a4fd83e3d6b7e1fb0f29b9ebd621b2110557151d)