Ferrocene: Difference between revisions
→With electrophiles: add formyation and rm redundant statement on dppf |
|||
| (40 intermediate revisions by 24 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Organometallic compound: Fe(II) sandwiched between two cyclopentadienyl rings}} |
{{Short description|Organometallic compound: Fe(II) sandwiched between two cyclopentadienyl rings}} |
||
{{chembox |
{{chembox |
||
|Verifiedfields=changed |
| Verifiedfields = changed |
||
|Watchedfields=changed |
| Watchedfields = changed |
||
|verifiedrevid=457631192 |
| verifiedrevid = 457631192 |
||
| ImageFileL1 = Ferrocene.svg |
|||
|ImageFile= |
|||
| |
| ImageFileR1 = Ferrocene-from-xtal-3D-balls.png |
||
| ImageFileL2 = Ferrocene 3d model 2.png |
|||
|ImageSizeL1= |
|||
| ImageFileR2 = Photo of Ferrocene (powdered).JPG |
|||
|ImageFileR1=Ferrocene-from-xtal-3D-balls.png |
|||
| PIN = Ferrocene<ref>{{cite book |author=[[International Union of Pure and Applied Chemistry]] |date=2014 |title=Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 |publisher=[[Royal Society of Chemistry|The Royal Society of Chemistry]] |pages=1041 |doi=10.1039/9781849733069 |isbn=978-0-85404-182-4}}</ref> |
|||
|ImageSizeR1= |
|||
| OtherNames = {{ubl|Dicyclopentadienyl iron|Bis(''η''<sup>5</sup>-cyclopentadienyl)iron|Iron(II) cyclopentadienide}} |
|||
|ImageFileL2=Ferrocene 3d model 2.png |
|||
| Section1 = {{Chembox Identifiers |
|||
|ImageFileR2=Photo of Ferrocene (powdered).JPG |
|||
|ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
|PIN=Ferrocene<ref>{{cite book |author=[[International Union of Pure and Applied Chemistry]] |date=2014 |title=Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 |publisher=[[Royal Society of Chemistry|The Royal Society of Chemistry]] |pages=1041 |doi=10.1039/9781849733069 |isbn=978-0-85404-182-4}}</ref> |
|||
|ChemSpiderID = 7329 |
|||
|OtherNames={{ubl|Dicyclopentadienyl iron|Bis(''η''<sup>5</sup>-cyclopentadienyl)iron|Iron(II) cyclopentadienide}} |
|||
|InChIKey = KTWOOEGAPBSYNW-UHFFFAOYAZ |
|||
|Section1={{Chembox Identifiers |
|||
| |
|StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
||
|StdInChI = 1S/2C5H5.Fe/c2*1-2-4-5-3-1;/h2*1-5H;/q2*-1;+2 |
|||
| ChemSpiderID = 7329 |
|||
|StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
| InChIKey = KTWOOEGAPBSYNW-UHFFFAOYAZ |
|||
|StdInChIKey = KTWOOEGAPBSYNW-UHFFFAOYSA-N |
|||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|||
|CASNo_Ref = {{cascite|correct|CAS}} |
|||
| StdInChI = 1S/2C5H5.Fe/c2*1-2-4-5-3-1;/h2*1-5H;/q2*-1;+2 |
|||
|CASNo = 102-54-5 |
|||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
|PubChem = 11985121 |
|||
| StdInChIKey = KTWOOEGAPBSYNW-UHFFFAOYSA-N |
|||
| |
|ChEBI_Ref = {{ebicite|changed|EBI}} |
||
| |
|ChEBI = 30672 |
||
|UNII_Ref = {{fdacite|correct|FDA}} |
|||
| PubChem = 11985121 |
|||
|UNII = U96PKG90JQ |
|||
| ChEBI_Ref = {{ebicite|changed|EBI}} |
|||
|SMILES = [CH-]1C=CC=C1.[CH-]1C=CC=C1.[Fe+2] |
|||
| ChEBI = 30672 |
|||
|Jmol = None |
|||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
|InChI = 1/2C5H5.Fe/c2*1-2-4-5-3-1;/h2*1-5H;/q2*-1;+2 |
|||
| UNII = U96PKG90JQ |
|||
| SMILES = [CH-]1C=CC=C1.[CH-]1C=CC=C1.[Fe+2] |
|||
| Jmol = [cH-]1cccc1.[Fe+2].[cH-]1cccc1<!-- altered from SMILES to show correct --> |
|||
| InChI = 1/2C5H5.Fe/c2*1-2-4-5-3-1;/h2*1-5H;/q2*-1;+2 |
|||
}} |
|||
|Section2={{Chembox Properties |
|||
| Formula = C<sub>10</sub>H<sub>10</sub>Fe |
|||
| MolarMass = 186.04 g/mol |
|||
| Appearance = light orange powder |
|||
| Odor = [[camphor]]-like |
|||
| Density = 1.107 g/cm<sup>3</sup> (0 °C), 1.490 g/cm<sup>3</sup> (20 °C)<ref>{{cite web|url=http://www.chemicalbook.com/ProductMSDSDetailCB1414721_EN.htm|title=Ferrocene (102-54-5) | work = ChemicalBook |access-date=3 February 2010}}</ref> |
|||
| MeltingPtC = 172.5 |
|||
| MeltingPt_ref = <ref>{{RubberBible86th|page=3.258|name-list-style=vanc}}</ref> |
|||
| BoilingPtC = 249 |
|||
| Solubility = Insoluble in water, soluble in most organic solvents |
|||
| LogP =2.04050 <ref name="chemsrc">{{Cite web|url=https://www.chemsrc.com/en/cas/102-54-5_311894.html|title=FERROCENE Material safety data sheet | work = ChemSrc }}</ref> |
|||
}} |
|||
|Section3={{Chembox Structure |
|||
| PointGroup = D<sub>5d</sub> / D<sub>5h</sub> / D<sub>5</sub> |
|||
| MolShape = Metallocene |
|||
| Dipole = No Permanent Dipole moment due to rapid Cp rotations<ref>{{Cite journal | vauthors = Mohammadi N, Ganesan A, Chantler CT, Wang F |doi = 10.1016/j.jorganchem.2012.04.009|title = Differentiation of ferrocene D5d and D5h conformers using IR spectroscopy|journal = Journal of Organometallic Chemistry|volume = 713|pages = 51–59|year = 2012 }}</ref> |
|||
}} |
}} |
||
| |
| Section2 = {{Chembox Properties |
||
|Formula = C<sub>10</sub>H<sub>10</sub>Fe |
|||
| GHSPictograms = |
|||
|MolarMass = 186.04 g/mol |
|||
| GHSSignalWord = |
|||
|Appearance = light orange powder |
|||
| HPhrases = |
|||
|Odor = [[camphor]]-like |
|||
| PPhrases = |
|||
|Density = 1.107 g/cm<sup>3</sup> (0 °C)<br>1.490 g/cm<sup>3</sup> (20 °C)<ref>{{cite web|url=http://www.chemicalbook.com/ProductMSDSDetailCB1414721_EN.htm|title=Ferrocene (102-54-5) | work = ChemicalBook |access-date=3 February 2010}}</ref> |
|||
| GHS_ref = |
|||
| |
|MeltingPtC = 172.5 |
||
|MeltingPt_ref = <ref>{{RubberBible86th|page=3.258|name-list-style=vanc}}</ref> |
|||
| NFPA-H = 3 |
|||
| |
|BoilingPtC = 249 |
||
|Solubility = Insoluble in water, soluble in most organic solvents |
|||
| NFPA_ref = <ref>{{cite web|title=Ferrocene MSDS|website=ScienceLab|url=http://www.sciencelab.com/msds.php?msdsId=9924047|access-date=2015-11-25|archive-url=https://web.archive.org/web/20151212034538/http://www.sciencelab.com/msds.php?msdsId=9924047|archive-date=2015-12-12|url-status=dead}}</ref> |
|||
|LogP =2.04050<ref name="chemsrc">{{Cite web|url=https://www.chemsrc.com/en/cas/102-54-5_311894.html|title=FERROCENE Material safety data sheet | work = ChemSrc }}</ref> |
|||
| FlashPt = |
|||
| AutoignitionPt = |
|||
| PEL = TWA 15 mg/m<sup>3</sup> (total) TWA 5 mg/m<sup>3</sup> (resp)<ref name=PGCH>{{PGCH|0205}}</ref> |
|||
| IDLH = N.D.<ref name=PGCH/> |
|||
| REL = TWA 10 mg/m<sup>3</sup> (total) TWA 5 mg/m<sup>3</sup> (resp)<ref name=PGCH/> |
|||
}} |
|||
|Section8={{Chembox Related |
|||
|OtherCompounds = [[cobaltocene]], [[nickelocene]], [[chromocene]], [[ruthenocene]], [[osmocene]], [[plumbocene]]}} |
|||
}} |
}} |
||
| Section3 = {{Chembox Structure |
|||
<!-- |Correct|https://doi.org/10.1016/j.jorganchem.2012.04.009 ???--> |
|||
|PointGroup = D<sub>5h</sub> (eclipsed)<br>D<sub>5d</sub> (staggered) |
|||
|MolShape = Sandwich structure with iron centre |
|||
}} |
|||
| Section4 = {{Chembox Hazards |
|||
|NFPA-F = 2 |
|||
|NFPA-H = 3 |
|||
|NFPA-R = 1 |
|||
|NFPA_ref = <ref>{{cite web|title=Ferrocene MSDS|website=ScienceLab|url=http://www.sciencelab.com/msds.php?msdsId=9924047|access-date=2015-11-25|archive-url=https://web.archive.org/web/20151212034538/http://www.sciencelab.com/msds.php?msdsId=9924047|archive-date=2015-12-12|url-status=dead}}</ref> |
|||
|PEL = TWA 15 mg/m<sup>3</sup> (total) TWA 5 mg/m<sup>3</sup> (resp)<ref name=PGCH>{{PGCH|0205}}</ref> |
|||
|IDLH = N.D.<ref name=PGCH/> |
|||
|REL = TWA 10 mg/m<sup>3</sup> (total) TWA 5 mg/m<sup>3</sup> (resp)<ref name=PGCH/> |
|||
}} |
|||
| Section5 = {{Chembox Related |
|||
|OtherCompounds = {{ubl|[[Metallocene]]|[[f-block metallocene]]|[[Actinocene]]|[[Actinocene#Characterised actinocenes|Thorocene]]|[[Actinocene#Characterised actinocenes|Protactinocene]]|[[Uranocene]]|[[Neptunocene]]|[[Plutonocene]]|[[Zirconocene]]|[[Vanadocene]]|[[Chromocene]]|[[Bis(benzene)chromium]]|[[Manganocene]]|[[Nickelocene]]|[[Cobaltocene]]|[[Ruthenocene]]|[[Rhodocene]]|[[Osmocene]]|[[Hassium#Experimental chemistry|Hassocene]]|[[Stannocene]]|[[Plumbocene]]}} |
|||
}} |
|||
}} |
|||
<!-- |Correct|https://doi.org/10.1016/j.jorganchem.2012.04.009 ?--> |
|||
'''Ferrocene''' is an [[organometallic chemistry|organometallic compound]] with the formula {{chem2|Fe(C5H5)2}}. The molecule is a [[Cyclopentadienyl complex|complex]] consisting of two [[Cyclopentadienyl anion|cyclopentadienyl]] rings |
'''Ferrocene''' is an [[organometallic chemistry|organometallic compound]] with the formula {{chem2|Fe(C5H5)2}}. The molecule is a [[Cyclopentadienyl complex|complex]] consisting of two [[Cyclopentadienyl anion|cyclopentadienyl]] rings sandwiching a central [[iron]] atom. It is an orange solid with a camphor-like odor that [[Sublimation (phase transition)|sublimes]] above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium [[cation]] {{chem2|Fe(C5H5)2(+)}}.<ref name="werner2012" /> Ferrocene and the ferrocenium cation are sometimes abbreviated as Fc and {{chem2|Fc+}} respectively. |
||
The first reported synthesis of ferrocene was in 1951. Its unusual stability puzzled chemists, and required the development of new theory to explain its formation and bonding. The discovery of ferrocene and its many [[Structural analog|analogues]], known as [[metallocene]]s, sparked excitement and led to a rapid growth in the discipline of [[organometallic chemistry]]. [[Geoffrey Wilkinson]] and [[Ernst Otto Fischer]], both of whom worked on elucidating the structure of ferrocene, later shared the 1973 [[Nobel Prize in Chemistry]] for their work on organometallic sandwich compounds. Ferrocene itself has no large-scale applications, but has found more niche uses in catalysis, as a fuel additive, and as a tool in undergraduate education. |
|||
The rapid growth of [[organometallic chemistry]] is often attributed to the excitement arising from the discovery of ferrocene and its many [[Structural analog|analogues]], such as [[metallocene]]s. |
|||
==History== |
==History== |
||
===Discovery=== |
===Discovery=== |
||
Ferrocene was discovered by accident |
Ferrocene was [[Role of chance in scientific discoveries|discovered by accident]] twice. The first known synthesis may have been made in the late 1940s by unknown researchers at [[Union Carbide]], who tried to pass hot cyclopentadiene vapor through an iron pipe. The vapor reacted with the pipe wall, creating a "yellow sludge" that clogged the pipe. Years later, a sample of the sludge that had been saved was obtained and analyzed by Eugene O. Brimm, shortly after reading Kealy and Pauson's article, and was found to consist of ferrocene.<ref name=werner2012/><ref name = Pauson2001 /> |
||
The second time was around 1950, when |
The second time was around 1950, when Samuel A. Miller, John A. Tebboth, and John F. Tremaine, researchers at [[BOC (company)|British Oxygen]], were attempting to synthesize amines from hydrocarbons and [[nitrogen]] in a modification of the [[Haber process]]. When they tried to react cyclopentadiene with nitrogen at 300 °C, at atmospheric pressure, they were disappointed to see the hydrocarbon react with some source of iron, yielding ferrocene. While they too observed its remarkable stability, they put the observation aside and did not publish it until after Pauson reported his findings.<ref name=werner2012/><ref name=miller/><ref name=laszloRmon/> Kealy and Pauson were later provided with a sample by Miller ''et al.'', who confirmed that the products were the same compound.<ref name = Pauson2001 /> |
||
In 1951, [[ |
In 1951, [[Peter L. Pauson]] and [[Thomas J. Kealy]] at [[Duquesne University]] attempted to prepare [[fulvalene]] ({{chem2|(C5H4)2}}) by oxidative dimerization of [[cyclopentadiene]] ({{chem2|C5H6}}). To that end, they reacted the [[Grignard reagent|Grignard]] compound [[cyclopentadienyl magnesium bromide]] in [[diethyl ether]] with [[iron(III) chloride|ferric chloride]] as an oxidizer.<ref name=werner2012/> However, instead of the expected fulvalene, they obtained a light orange powder of "remarkable stability", with the formula {{chem2|C10H10Fe}}.<ref name = Pauson2001 /><ref name=pauson/> |
||
===Determining the structure=== |
===Determining the structure=== |
||
| Line 86: | Line 76: | ||
Pauson and Kealy conjectured that the compound had two cyclopentadienyl groups, each with a single covalent bond from the saturated carbon atom to the iron atom.<ref name=werner2012/> However, that structure was inconsistent with then-existing bonding models and did not explain the unexpected stability of the compound, and chemists struggled to find the correct structure.<ref name=laszloRmon/><ref name=federman/> |
Pauson and Kealy conjectured that the compound had two cyclopentadienyl groups, each with a single covalent bond from the saturated carbon atom to the iron atom.<ref name=werner2012/> However, that structure was inconsistent with then-existing bonding models and did not explain the unexpected stability of the compound, and chemists struggled to find the correct structure.<ref name=laszloRmon/><ref name=federman/> |
||
The structure was deduced and reported independently by three groups in 1952.<ref name=werner2008/> [[Robert Burns Woodward]], [[Geoffrey Wilkinson]], et al. deduced observe that the compound was diamagnetic and nonpolar.<ref name="wilk52" /> A few months later they described its reactions as being typical of aromatic compounds such as [[benzene]].<ref>{{Cite journal |last=Woodward |first=R. B. |last2=Rosenblum |first2=M. |last3=Whiting |first3=M. C. |date=1952-06-02 |title=A NEW AROMATIC SYSTEM |url=https://pubs.acs.org/doi/abs/10.1021/ja01133a543 |journal=Journal of the American Chemical Society |language=en |volume=74 |issue=13 |pages=3458–3459 |doi=10.1021/ja01133a543 |issn=0002-7863}}</ref> The name ferrocene was coined by Mark Whiting, a postdoc with Woodward.<ref>{{Cite web |last=Sutton |first=Mike |date=2023-09-27 |title=Fifty years since the ferrocene furore |url=https://www.chemistryworld.com/features/fifty-years-since-the-ferrocene-furore/4018098.article |access-date=2024-11-02 |website=Chemistry World |language=en}}</ref>. [[Ernst Otto Fischer]] and Wolfgang Pfab also noted ferrocene's diamagneticity and high symmetry. They also synthesize [[nickelocene]] and [[cobaltocene]] and confirmed they had the same structure.<ref name="fischer" /> Fischer described the structure as ''Doppelkegelstruktur'' ("double-cone structure"), although the term "sandwich" came to be preferred by British and American chemists.<ref name="okuda" /> Philip Frank Eiland and Raymond Pepinsky confirmed the structure through [[X-ray crystallography]] and later by [[Nuclear magnetic resonance|NMR]] spectroscopy.<ref name="laszloRmon" /><ref name="eiland52" /><ref name="dunitz53" /><ref name="dunitz56" /> |
|||
The structure was deduced and reported independently by three groups in 1952:<ref name=werner2008/> |
|||
*[[Robert Burns Woodward|Woodward]] and [[Geoffrey Wilkinson|Wilkinson]] deduced it by observing that ferrocene underwent reactions typical of aromatic compounds such as [[benzene]]<ref name=wilk52/> |
|||
*[[Ernst Otto Fischer|E. Fischer]] deduced the structure (which he called "double cone") and also synthesized other [[metallocene]]s such as [[nickelocene]] and [[cobaltocene]].<ref name=fischer/><ref name=fischer2/><ref name=okuda/> |
|||
*[[P. F. Eiland]] and [[R. Pepinsky]] confirmed the structure through [[X-ray crystallography]] and later by [[Nuclear magnetic resonance|NMR]].<ref name=laszloRmon/><ref name=eiland52/><ref name=dunitz53/><ref name=dunitz56/> |
|||
The "sandwich" structure of ferrocene was shockingly novel and led to intensive theoretical studies. Application of [[molecular orbital theory]] with the assumption of a Fe<sup>2+</sup> centre between two [[cyclopentadienide]] anions {{chem2|C5H5(-)}} resulted in the successful [[Dewar–Chatt–Duncanson model]], allowing correct prediction of the geometry of the molecule as well as explaining its remarkable stability.<ref name=mingos/><ref name=mehr/> |
|||
===Understanding the structure=== |
|||
The "sandwich" structure of ferrocene was shockingly novel, and required new theory to explain. Application of [[molecular orbital theory]] with the assumption of a Fe<sup>2+</sup> centre between two [[cyclopentadienide]] anions {{chem2|C5H5(-)}} resulted in the successful [[Dewar–Chatt–Duncanson model]], allowing correct prediction of the geometry of the molecule as well as explaining its remarkable stability.<ref name=mingos/><ref name=mehr/> |
|||
===Impact=== |
===Impact=== |
||
The discovery of ferrocene was considered so significant that Wilkinson and Fischer shared the 1973 [[Nobel Prize in Chemistry]] "for their pioneering work, performed independently, on the chemistry of the organometallic, called [[sandwich compound]]s".<ref>{{Cite web |url= http://nobelprize.org/nobel_prizes/chemistry/laureates/1973/ |title= The Nobel Prize in Chemistry 1973 |publisher= [[Nobel Foundation]] |access-date= 12 September 2010}}</ref> |
|||
Ferrocene was not the first organometallic compound to be discovered. [[Zeise's salt]] {{chem2|K[PtCl3(C2H4)]*H2O}} was reported in 1831,<ref name=zeise/><ref name=hunt/> [[Ludwig Mond|Mond's]] discovery of Ni(CO)<sub>4</sub> occurred in 1888,<ref name=leigh/> and [[organolithium compound]]s were developed in the 1930s.<ref name=eisch2002/> However, it can be argued that it was ferrocene's discovery that began [[organometallic chemistry]] as a separate area of chemistry. It also led to an explosion of interest in compounds of [[d-block]] metals with hydrocarbons. |
|||
The discovery was considered so significant that Wilkinson and Fischer shared the 1973 [[Nobel Prize in Chemistry|Nobel Prize]] for Chemistry "for their pioneering work, performed independently, on the chemistry of the organometallic, so called [[sandwich compound]]s".<ref>{{Cite web |url= http://nobelprize.org/nobel_prizes/chemistry/laureates/1973/ |title= The Nobel Prize in Chemistry 1973 |publisher= [[Nobel Foundation]] |access-date= 12 September 2010}}</ref> |
|||
==Structure and bonding== |
==Structure and bonding== |
||
[[Mössbauer spectroscopy]] indicates that the iron center in ferrocene should be assigned the +2 oxidation state. Each cyclopentadienyl (Cp) ring should then be allocated a single negative charge. |
[[Mössbauer spectroscopy]] indicates that the iron center in ferrocene should be assigned the +2 oxidation state. Each cyclopentadienyl (Cp) ring should then be allocated a single negative charge. Thus ferrocene could be described as iron(II) bis([[cyclopentadienide]]), {{chem2|Fe(2+)[C5H5(-)]2}}. |
||
Each ring has six π-electrons, which makes them [[Aromaticity|aromatic]] according to [[Hückel's rule]]. These π-electrons are then shared with the metal via covalent bonding. Since Fe<sup>2+</sup> has six ''d''-electrons, the complex attains an [[18-electron rule|18-electron]] configuration, which accounts for its stability. In modern notation, this sandwich structural model of the ferrocene molecule is denoted as {{chem2|Fe(''η''^{5}\-C5H5)2}}, where ''η'' denotes [[hapticity]], the number of atoms through which each ring binds. |
|||
The carbon–carbon bond distances around each five-membered ring are all 1.40 Å, and |
The carbon–carbon bond distances around each five-membered ring are all 1.40 Å, and all Fe–C bond distances are 2.04 Å. From room temperature down to 164 K, [[X-ray crystallography]] yields the monoclinic space group; the cyclopentadienide rings are a staggered conformation, resulting in a centrosymmetric molecule, with [[symmetry group]] D<sub>5d</sub>.<ref name=eiland52/> However, below 110 K, ferrocene crystallizes in an orthorhombic crystal lattice in which the Cp rings are ordered and eclipsed, so that the molecule has symmetry group D<sub>5h</sub>.<ref name=seiler/> In the gas phase, [[electron diffraction]]<ref name=haal68/> and computational studies<ref name=coriani/> show that the Cp rings are eclipsed. While ferrocene has no permanent dipole moment at room temperature, between 172.8 and 163.5 K the molecule exhibits an "incommensurate modulation", breaking the D<sub>5</sub> symmetry and acquiring an electric dipole.<ref>{{Cite journal |last=Katrusiak |first=Andrzej |last2=Rusek |first2=Michalina |last3=Dušek |first3=Michal |last4=Petříček |first4=Václav |last5=Szafrański |first5=Marek |date=2023-04-06 |title=Dipole-Moment Modulation in New Incommensurate Ferrocene |url=https://pubs.acs.org/doi/10.1021/acs.jpclett.3c00215 |journal=The Journal of Physical Chemistry Letters |language=en |volume=14 |issue=13 |pages=3111–3119 |doi=10.1021/acs.jpclett.3c00215 |issn=1948-7185 |pmc=10084461 |pmid=36951481}}</ref> |
||
The Cp rings rotate with a low barrier about the Cp<sub>(centroid)</sub>–Fe–Cp<sub>(centroid)</sub> axis, as observed by measurements on substituted derivatives of ferrocene using <sup>1</sup>H and <sup>13</sup>C [[nuclear magnetic resonance]] spectroscopy. |
The Cp rings rotate with a low barrier about the Cp<sub>(centroid)</sub>–Fe–Cp<sub>(centroid)</sub> axis, as observed by measurements on substituted derivatives of ferrocene using <sup>1</sup>H and <sup>13</sup>C [[nuclear magnetic resonance]] spectroscopy. For example, methylferrocene (CH<sub>3</sub>C<sub>5</sub>H<sub>4</sub>FeC<sub>5</sub>H<sub>5</sub>) exhibits a singlet for the C<sub>5</sub>H<sub>5</sub> ring.<ref>{{cite journal | vauthors = Abel EW, Long NJ, Orrell KG, Osborne AG, Šik V |title = Dynamic NMR studies of ring rotation in substituted ferrocenes and ruthenocenes |journal = [[Journal of Organometallic Chemistry|J. Org. Chem.]] |year = 1991 |volume = 403 |issue=1–2 |pages = 195–208 |doi = 10.1016/0022-328X(91)83100-I}}</ref> |
||
In solution, and at room temperature, eclipsed D<sub>5h</sub> ferrocene was determined to dominate over the staggered D<sub>5d</sub> conformer, as suggested by both [[Fourier-transform infrared spectroscopy]] and [[Density functional theory|DFT]] calculations.<ref>{{Cite journal |last1=Wang |first1=Feng |last2=Mohammadi |first2=Narges |last3=Best |first3=Stephen P. |last4=Appadoo |first4=Dominique |last5=Chantler |first5=Christopher T. |title=Dominance of eclipsed ferrocene conformer in solutions revealed by the IR spectra between 400 and 500 cm-1 |url=https://linkinghub.elsevier.com/retrieve/pii/S0969806X21002401 |journal=Radiation Physics and Chemistry |year=2021 |language=en |volume=188 |pages=109590 |doi=10.1016/j.radphyschem.2021.109590|bibcode=2021RaPC..18809590W}}</ref> |
|||
==Synthesis== |
==Synthesis== |
||
=== |
===Early methods=== |
||
The first reported syntheses of ferrocene were nearly simultaneous. Pauson and Kealy synthesised ferrocene using iron(III) chloride and cyclopentadienyl magnesium bromide.<ref name=pauson/> A [[redox|redox reaction]] produces iron(II) chloride. The formation of fulvalene, the intended outcome does not occur.<ref name = Pauson2001>{{cite journal|title = Ferrocene—how it all began| vauthors = Pauson PL |author-link = Peter Pauson|journal = [[Journal of Organometallic Chemistry]]|volume = 637–639|year = 2001|pages = 3–6|doi = 10.1016/S0022-328X(01)01126-3}}</ref> |
|||
Industrially, ferrocene is synthesized by the reaction of [[iron(II)]] [[ethoxide]] with cyclopentadiene;<ref>{{Cite web|url=https://pubchem.ncbi.nlm.nih.gov/compound/7611|title = Iron, bis(eta5-2,4-cyclopentadien-1-yl)-}}</ref> the iron(II) ethoxide needed is produced by the [[electrochemical]] [[oxidation]] of metallic iron in anhydrous [[ethanol]]. Since the reaction between iron(II) ethoxide and cyclopentadiene produces ethanol as a byproduct, the ethanol effectively serves as a [[catalyst]] for the overall reaction, with the net reaction being Fe + 2C<sub>5</sub>H<sub>6</sub> → H<sub>2</sub> + Fe(C<sub>5</sub>H<sub>5</sub>)<sub>2</sub> (also [[Ferrocene#Gas-metal reaction|see below]]) |
|||
===Via Grignard reagent=== |
|||
The first reported syntheses of ferrocene were nearly simultaneous. Pauson and Kealy synthesised ferrocene using iron(III) chloride and a Grignard reagent, cyclopentadienyl magnesium bromide. Iron(III) chloride is suspended in [[anhydrous]] diethyl ether and added to the Grignard reagent.<ref name=pauson/> A [[redox|redox reaction]] occurs, forming the cyclopentadienyl [[radical (chemistry)|radical]] and iron(II) ions. Dihydrofulvalene is produced by [[radical-radical recombination]] while the iron(II) reacts with the Grignard reagent to form ferrocene. Oxidation of dihydrofulvalene to fulvalene with iron(III), the outcome sought by Kealy and Pauson, does not occur.<ref name = Pauson2001>{{cite journal|title = Ferrocene—how it all began| vauthors = Pauson PL |author-link = Peter Pauson|journal = [[Journal of Organometallic Chemistry]]|volume = 637–639|year = 2001|pages = 3–6|doi = 10.1016/S0022-328X(01)01126-3}}</ref> |
|||
:[[File:Kealy and Pauson synthesis of ferrocene v2.jpg|600px]] |
:[[File:Kealy and Pauson synthesis of ferrocene v2.jpg|600px]] |
||
===Gas-metal reaction=== |
|||
[[File:Miller Ferrocen Synthese.svg|thumb|right|300px|The Miller ''et al.''<ref name=miller/> approach to ferrocene]] |
|||
The other early synthesis of ferrocene was by Miller ''et al.'',<ref name=miller/> who reacted metallic iron directly with [[gas]]-phase cyclopentadiene at elevated temperature.<ref>{{cite journal | vauthors = Wilkinson G, Pauson PL, Cotton FA |author-link1=Geoffrey Wilkinson |author-link3=F. Albert Cotton |doi=10.1021/ja01636a080|title=Bis-cyclopentadienyl Compounds of Nickel and Cobalt|year=1954|journal=[[J. Am. Chem. Soc.]]|volume=76|pages=1970–1974|issue=7}}</ref> An approach using [[iron pentacarbonyl]] was also reported.<ref>{{cite book | vauthors = Wilkinson G, Cotton FA |author-link1=Geoffrey Wilkinson |author-link2=F. Albert Cotton |doi=10.1002/9780470166024.ch1|year=1959 |chapter=Cyclopentadienyl and Arene Metal Compounds|title=Progress in Inorganic Chemistry|volume=1|pages=1–124|isbn=978-0-470-16602-4}}</ref> |
|||
[[File:Miller Ferrocen Synthese.svg|thumb|300px|The Miller ''et al.''<ref name=miller/> approach to ferrocene|left]] |
|||
:Fe(CO)<sub>5</sub> + 2 C<sub>5</sub>H<sub>6</sub>(g) → Fe(C<sub>5</sub>H<sub>5</sub>)<sub>2</sub> + 5 CO(g) + H<sub>2</sub>(g) |
|||
Another early synthesis of ferrocene was by Miller ''et al.'',<ref name=miller/> who treated metallic iron with [[gas]]eous cyclopentadiene at elevated temperature.<ref>{{cite journal | vauthors = Wilkinson G, Pauson PL, Cotton FA |author-link1=Geoffrey Wilkinson |author-link3=F. Albert Cotton |doi=10.1021/ja01636a080|title=Bis-cyclopentadienyl Compounds of Nickel and Cobalt|year=1954|journal=[[J. Am. Chem. Soc.]]|volume=76|pages=1970–1974|issue=7}}</ref> An approach using [[iron pentacarbonyl]] was also reported.<ref>{{cite book | vauthors = Wilkinson G, Cotton FA |author-link1=Geoffrey Wilkinson |author-link2=F. Albert Cotton |doi=10.1002/9780470166024.ch1|year=1959 |chapter=Cyclopentadienyl and Arene Metal Compounds|title=Progress in Inorganic Chemistry|volume=1|pages=1–124|isbn=978-0-470-16602-4}}</ref> |
|||
:Fe(CO)<sub>5</sub> + 2 C<sub>5</sub>H<sub>6</sub> → Fe(C<sub>5</sub>H<sub>5</sub>)<sub>2</sub> + 5 CO + H<sub>2</sub> |
|||
===Via alkali cyclopentadienide=== |
===Via alkali cyclopentadienide=== |
||
| Line 135: | Line 118: | ||
:2 C<sub>5</sub>H<sub>6</sub> + 2 (CH<sub>3</sub>CH<sub>2</sub>)<sub>2</sub>NH + FeCl<sub>2</sub> → Fe(C<sub>5</sub>H<sub>5</sub>)<sub>2</sub> + 2 (CH<sub>3</sub>CH<sub>2</sub>)<sub>2</sub>NH<sub>2</sub>Cl |
:2 C<sub>5</sub>H<sub>6</sub> + 2 (CH<sub>3</sub>CH<sub>2</sub>)<sub>2</sub>NH + FeCl<sub>2</sub> → Fe(C<sub>5</sub>H<sub>5</sub>)<sub>2</sub> + 2 (CH<sub>3</sub>CH<sub>2</sub>)<sub>2</sub>NH<sub>2</sub>Cl |
||
Direct transmetalation can also be used to prepare ferrocene from other metallocenes, such as [[manganocene]]:<ref>{{cite journal | vauthors = Wilkinson G, Cotton FA, Birmingham JM |author-link1= Geoffrey Wilkinson |
Direct transmetalation can also be used to prepare ferrocene from some other metallocenes, such as [[manganocene]]:<ref>{{cite journal | vauthors = Wilkinson G, Cotton FA, Birmingham JM |author-link1= Geoffrey Wilkinson |year= 1956|title= On manganese cyclopentadienide and some chemical reactions of neutral bis-cyclopentadienyl metal compounds|journal= [[J. Inorg. Nucl. Chem.]]|volume= 2|issue= 2|pages= 95–113|doi=10.1016/0022-1902(56)80004-3}}</ref> |
||
:FeCl<sub>2</sub> + Mn(C<sub>5</sub>H<sub>5</sub>)<sub>2</sub> → MnCl<sub>2</sub> + Fe(C<sub>5</sub>H<sub>5</sub>)<sub>2</sub> |
:FeCl<sub>2</sub> + Mn(C<sub>5</sub>H<sub>5</sub>)<sub>2</sub> → MnCl<sub>2</sub> + Fe(C<sub>5</sub>H<sub>5</sub>)<sub>2</sub> |
||
<!-- |
|||
:[[File:Synthesis of acetylferrocene from dicyclopentadiene.png|frameless|upright=3.65]] |
|||
--> |
|||
==Properties== |
==Properties== |
||
[[File:Ferrocen.jpg|thumb|right|Crystals of ferrocene after purification by vacuum sublimation]] |
[[File:Ferrocen.jpg|thumb|right|Crystals of ferrocene after purification by vacuum sublimation]] |
||
Ferrocene is an [[air]]-stable orange solid with a camphor-like odor. |
Ferrocene is an [[air]]-stable orange solid with a camphor-like odor. As expected for a symmetric, uncharged species, ferrocene is soluble in normal organic solvents, such as benzene, but is insoluble in water. It is stable to temperatures as high as 400 °C.<ref>{{cite book| vauthors = Solomons G, Craig F |title=Organic Chemistry |edition=9th |location=USA |publisher=John Wiley & Sons|date=2006}}</ref> |
||
Ferrocene readily [[Sublimation (phase transition)|sublimes]], especially upon heating in a vacuum. |
Ferrocene readily [[Sublimation (phase transition)|sublimes]], especially upon heating in a vacuum. Its vapor pressure is about 1 [[Pascal (unit)|Pa]] at 25 °C, 10 Pa at 50 °C, 100 Pa at 80 °C, 1000 Pa at 116 °C, and 10,000 Pa (nearly 0.1 [[atmosphere (unit)|atm]]) at 162 °C.<ref>{{cite journal | vauthors = Monte MJ, Santos LM, Fulem M, Fonseca JM, Sousa CA |doi=10.1021/je050502y|title=New Static Apparatus and Vapor Pressure of Reference Materials: Naphthalene, Benzoic Acid, Benzophenone, and Ferrocene|year=2006 |journal=Journal of Chemical & Engineering Data |volume=51|page=757|issue=2}}</ref><ref name=fulem2013>{{cite journal | vauthors = Fulem M, Růžička K, Červinka C, Rocha MA, Santos LM, Berg RF | year = 2013 | title = Recommended vapor pressure and thermophysical data for ferrocene | journal = Journal of Chemical Thermodynamics | volume = 57 | pages = 530–540 | doi = 10.1016/j.jct.2012.07.023 }}</ref> |
||
==Reactions== |
==Reactions== |
||
===With electrophiles=== |
===With electrophiles=== |
||
Ferrocene undergoes many reactions characteristic of aromatic compounds, enabling the preparation of substituted derivatives. A common undergraduate experiment is the [[Friedel–Crafts reaction]] of ferrocene with [[acetic anhydride]] (or [[acetyl chloride]]) in the presence of [[phosphoric acid]] as a catalyst. Under conditions for a [[Mannich reaction]], ferrocene gives [[N,N-Dimethylaminomethylferrocene|N,N-dimethylaminomethylferrocene]]. |
Ferrocene undergoes many reactions characteristic of aromatic compounds, enabling the preparation of substituted derivatives. A common undergraduate experiment is the [[Friedel–Crafts reaction]] of ferrocene with [[acetic anhydride]] (or [[acetyl chloride]]) in the presence of [[phosphoric acid]] as a catalyst. Under conditions for a [[Mannich reaction]], ferrocene gives [[N,N-Dimethylaminomethylferrocene|N,N-dimethylaminomethylferrocene]]. |
||
[[Image:FcGen'l.png|400px|thumb|center|Important reactions of ferrocene with electrophiles and other reagents |
[[Image:FcGen'l.png|400px|thumb|center|Important reactions of ferrocene with electrophiles and other reagents]] |
||
<!--- Ferrocene itself can be used as the backbone of a ligand, e.g. [[1,1'-bis(diphenylphosphino)ferrocene]] (dppf). Ferrocene can itself be oxidized to the ferrocenium cation (Fc<sup>+</sup>); the ferrocene/ferrocenium couple is often used as a reference in electrochemistry.<ref name=federman/>--> |
|||
Ferrocene can itself be oxidized to the ferrocenium cation (Fc<sup>+</sup>); the ferrocene/ferrocenium couple is often used as a reference in electrochemistry.<ref name=federman/> |
|||
<!----> |
|||
<!---It is an [[aromaticity|aromatic substance]] and undergoes [[substitution reaction]]s rather than [[addition reaction]]s on the cyclopentadienyl ligands. For example, [[Friedel-Crafts reaction#Friedel-Crafts acylation|Friedel-Crafts acylation]] of ferrocene with [[acetic anhydride]] yields [[acetylferrocene]]<ref>{{cite journal| vauthors = Bozak RE |title = Acetylation of Ferrocene: A Chromatography Experiment for Elementary Organic Laboratory|journal = [[J. Chem. Educ.]]|year = 1966|volume = 43|issue = 2|page = 73|doi = 10.1021/ed043p73|bibcode = 1966JChEd..43...73B}}</ref> just as acylation of benzene yields [[acetophenone]] under similar conditions. |
|||
It is an [[aromaticity|aromatic substance]] and undergoes [[substitution reaction]]s rather than [[addition reaction]]s on the cyclopentadienyl ligands. For example, [[Friedel-Crafts reaction#Friedel-Crafts acylation|Friedel-Crafts acylation]] of ferrocene with [[acetic anhydride]] yields [[acetylferrocene]]<ref>{{cite journal| vauthors = Bozak RE |title = Acetylation of Ferrocene: A Chromatography Experiment for Elementary Organic Laboratory|journal = [[J. Chem. Educ.]]|year = 1966|volume = 43|issue = 2|page = 73|doi = 10.1021/ed043p73|bibcode = 1966JChEd..43...73B}}</ref> just as acylation of benzene yields [[acetophenone]] under similar conditions. [[Vilsmeier-Haack reaction]] (formylation) using formylanilide and [[phosphorus oxychloride]] gives [[ferrocenecarboxaldehyde]]. Diformylation does not occur readily, showing the electronic communication between the two rings.<ref name=Rausch>{{cite journal |doi=10.1139/v63-182 |title=Metallocene Chemistry—A Decade of Progress |date=1963 |last1=Rausch |first1=M. D. |journal=Canadian Journal of Chemistry |volume=41 |issue=5 |pages=1289–1314 }}</ref> |
|||
--> |
|||
Protonation of ferrocene allows isolation of [Cp<sub>2</sub>FeH]PF<sub>6</sub>.<ref>{{cite journal | vauthors = Malischewski M, Seppelt K, Sutter J, Heinemann FW, Dittrich B, Meyer K | title = Protonation of Ferrocene: A Low-Temperature X-ray Diffraction Study of [Cp<sub>2</sub> FeH](PF<sub>6</sub> ) Reveals an Iron-Bound Hydrido Ligand | journal = Angewandte Chemie | volume = 56 | issue = 43 | pages = 13372–13376 | date = October 2017 | pmid = 28834022 | doi = 10.1002/anie.201704854 }}</ref> |
|||
Protonation of ferrocene allows isolation of [Cp<sub>2</sub>FeH]PF<sub>6</sub>.<ref>{{cite journal | vauthors = Malischewski M, Seppelt K, Sutter J, Heinemann FW, Dittrich B, Meyer K | title = Protonation of Ferrocene: A Low-Temperature X-ray Diffraction Study of [Cp<sub>2</sub> FeH](PF<sub>6</sub> ) Reveals an Iron-Bound Hydrido Ligand | journal = Angewandte Chemie | volume = 56 | issue = 43 | pages = 13372–13376 | date = October 2017 | pmid = 28834022 | doi = 10.1002/anie.201704854}}</ref> |
|||
In the presence of [[aluminium chloride]] Me<sub>2</sub>NPCl<sub>2</sub> and ferrocene react to give ferrocenyl dichlorophosphine,<ref>{{cite journal |title = Ferrocene derivatives. 27. Ferrocenyldimethylphosphine | vauthors = Knox GR, Pauson PL, Willison D |author-link2=Peter Pauson |journal = Organometallics |volume = 11 |issue = 8 |pages = 2930–2933 |year = 1992 |doi = 10.1021/om00044a038 }}</ref> whereas treatment with [[dichlorophenylphosphine|phenyldichlorophosphine]] under similar conditions forms ''P'',''P''-diferrocenyl-''P''-phenyl phosphine.<ref>{{cite journal | vauthors = Sollott GP, Mertwoy HE, Portnoy S, Snead JL |title = Unsymmetrical Tertiary Phosphines of Ferrocene by Friedel–Crafts Reactions. I. Ferrocenylphenylphosphines |journal = [[J. Org. Chem.]] |year = 1963 |volume = 28 |pages = 1090–1092 |doi = 10.1021/jo01039a055 |issue = 4 }}</ref> |
In the presence of [[aluminium chloride]], Me<sub>2</sub>NPCl<sub>2</sub> and ferrocene react to give ferrocenyl dichlorophosphine,<ref>{{cite journal |title = Ferrocene derivatives. 27. Ferrocenyldimethylphosphine | vauthors = Knox GR, Pauson PL, Willison D |author-link2=Peter Pauson |journal = Organometallics |volume = 11 |issue = 8 |pages = 2930–2933 |year = 1992 |doi = 10.1021/om00044a038 }}</ref> whereas treatment with [[dichlorophenylphosphine|phenyldichlorophosphine]] under similar conditions forms ''P'',''P''-diferrocenyl-''P''-phenyl phosphine.<ref>{{cite journal | vauthors = Sollott GP, Mertwoy HE, Portnoy S, Snead JL |title = Unsymmetrical Tertiary Phosphines of Ferrocene by Friedel–Crafts Reactions. I. Ferrocenylphenylphosphines |journal = [[J. Org. Chem.]] |year = 1963 |volume = 28 |pages = 1090–1092 |doi = 10.1021/jo01039a055 |issue = 4 }}</ref> |
||
Ferrocene reacts with [[Phosphorus pentasulfide|P<sub>4</sub>S<sub>10</sub>]] forms a diferrocenyl-dithiadiphosphetane disulfide.<ref>{{cite journal |title = 2,4-Diferrocenyl-1,3-dithiadiphosphetane 2,4-disulfide; structure and reactions with catechols and [PtCl<sub>2</sub>(PR<sub>3</sub>)<sub>2</sub>](R = Et or Bun) | vauthors = ((St J Foreman MR)), Alexandra MZ, Slawin AM, Woollins JD |journal = [[Dalton Transactions|J. Chem. Soc., Dalton Trans.]] |issue = 18 |year = 1996 |pages = 3653–3657 |doi = 10.1039/DT9960003653}}</ref> |
Ferrocene reacts with [[Phosphorus pentasulfide|P<sub>4</sub>S<sub>10</sub>]] forms a diferrocenyl-dithiadiphosphetane disulfide.<ref>{{cite journal |title = 2,4-Diferrocenyl-1,3-dithiadiphosphetane 2,4-disulfide; structure and reactions with catechols and [PtCl<sub>2</sub>(PR<sub>3</sub>)<sub>2</sub>](R = Et or Bun) | vauthors = ((St J Foreman MR)), Alexandra MZ, Slawin AM, Woollins JD |journal = [[Dalton Transactions|J. Chem. Soc., Dalton Trans.]] |issue = 18 |year = 1996 |pages = 3653–3657 |doi = 10.1039/DT9960003653}}</ref> |
||
===Lithiation=== |
===Lithiation=== |
||
Ferrocene reacts with [[butyllithium]] to give [[1,1'-Dilithioferrocene|1,1′-dilithioferrocene]], which is a versatile [[nucleophile]]. In combination with butyllithiium, [[Tert-Butyllithium| |
Ferrocene reacts with [[butyllithium]] to give [[1,1'-Dilithioferrocene|1,1′-dilithioferrocene]], which is a versatile [[nucleophile]]. In combination with butyllithiium, [[Tert-Butyllithium|''tert''-butyllithium]] produces monolithioferrocene.<ref>{{cite journal |author=Busacca |first=Carl A. |last2=Eriksson |first2=Magnus C. |last3=Haddad |first3=Nizar |last4=Han |first4=Z. Steve |last5=Lorenz |first5=Jon C. |last6=Qu |first6=Bo |last7=Zeng |first7=Xingzhong |last8=Senanayake |first8=Chris H. |year=2013 |title=Practical Synthesis of Di-tert-Butyl-Phosphinoferrocene |journal=Organic Syntheses |volume=90 |page=316 |doi=10.15227/orgsyn.090.0316 |doi-access=free}}</ref> |
||
=== Redox chemistry === |
=== Redox chemistry === |
||
Ferrocene undergoes a one-electron oxidation at around 0.4 V versus a [[saturated calomel electrode]] (SCE), becoming '''ferrocenium'''. This reversible oxidation has been used as standard in electrochemistry as Fc<sup>+</sup>/Fc = 0.64 V versus the [[standard hydrogen electrode]] |
Ferrocene undergoes a one-electron oxidation at around 0.4 V versus a [[saturated calomel electrode]] (SCE), becoming '''ferrocenium'''. This reversible oxidation has been used as standard in electrochemistry as Fc<sup>+</sup>/Fc = 0.64 V versus the [[standard hydrogen electrode]],<ref>{{cite journal | vauthors = Cardona CM, Li W, Kaifer AE, Stockdale D, Bazan GC | title = Electrochemical considerations for determining absolute frontier orbital energy levels of conjugated polymers for solar cell applications | journal = Advanced Materials | volume = 23 | issue = 20 | pages = 2367–2371 | date = May 2011 | pmid = 21462372 | doi = 10.1002/adma.201004554 | bibcode = 2011AdM....23.2367C | s2cid = 40766788 }}</ref> however other values have been reported.<ref>{{citation|surname1=Vitaly V Pavlishchuk, Anthony W Addison|periodical=Inorganica Chimica Acta|title=Conversion constants for redox potentials measured versus different reference electrodes in acetonitrile solutions at 25°C|volume=298|issue=1|at=pp. 97–102|date=January 2000 |language=German |doi=10.1016/S0020-1693(99)00407-7|url=https://linkinghub.elsevier.com/retrieve/pii/S0020169399004077|access-date=2022-07-26 |
||
}}</ref> [[Ferrocenium tetrafluoroborate]] is a common reagent.<ref>{{cite journal | vauthors = Connelly NG, Geiger WE | title = Chemical Redox Agents for Organometallic Chemistry | journal = Chemical Reviews | volume = 96 | issue = 2 | pages = 877–910 | date = March 1996 | pmid = 11848774 | doi = 10.1021/cr940053x }}</ref> The remarkably reversible oxidation-reduction behaviour has been extensively used to control electron-transfer processes in electrochemical<ref>{{cite journal | vauthors = Sirbu D, Turta C, Gibson EA, Benniston AC | title = The ferrocene effect: enhanced electrocatalytic hydrogen production using meso-tetraferrocenyl porphyrin palladium(II) and copper(II) complexes | journal = Dalton Transactions | volume = 44 | issue = 33 | pages = 14646–14655 | date = September 2015 | pmid = 26213204 | doi = 10.1039/C5DT02191J | url = https://eprint.ncl.ac.uk/fulltext.aspx?url=214473/2DD72927-25BC-4809-8636-5FDB014D7CB0.pdf&pub_id=214473 }}</ref><ref>{{cite journal | vauthors = Lennox AJ, Nutting JE, Stahl SS | title = Selective electrochemical generation of benzylic radicals enabled by ferrocene-based electron-transfer mediators | journal = Chemical Science | volume = 9 | issue = 2 | pages = 356–361 | date = January 2018 | pmid = 29732109 | pmc = 5909123 | doi = 10.1039/C7SC04032F | doi-access = free }}</ref> and photochemical<ref>{{Cite journal| vauthors = Dannenberg JJ, Richards JH |date=1965-04-01|title=Photosensitization by Ferrocene. Photochemistry of Higher Electronic Excited States|journal=Journal of the American Chemical Society|volume=87|issue=7|pages=1626–1627|doi=10.1021/ja01085a048|issn=0002-7863}}</ref><ref>{{Cite journal| vauthors = Sirbu D, Turta C, Benniston AC, Abou-Chahine F, Lemmetyinen H, Tkachenko NV, Wood C, Gibson E | display-authors = 6 |date=2014-05-23|title=Synthesis and properties of a meso- tris–ferrocene appended zinc(II) porphyrin and a critical evaluation of its dye sensitised solar cell (DSSC) performance |journal=RSC Advances|language=en|volume=4|issue=43|pages=22733–22742|doi=10.1039/C4RA03105A|bibcode=2014RSCAd...422733S |issn=2046-2069| url = https://eprint.ncl.ac.uk/fulltext.aspx?url=199647/CED25CA5-111E-4500-8188-DCFC5D4D7190.pdf&pub_id=199647 |
}}</ref> [[Ferrocenium tetrafluoroborate]] is a common reagent.<ref>{{cite journal | vauthors = Connelly NG, Geiger WE | title = Chemical Redox Agents for Organometallic Chemistry | journal = Chemical Reviews | volume = 96 | issue = 2 | pages = 877–910 | date = March 1996 | pmid = 11848774 | doi = 10.1021/cr940053x }}</ref> The remarkably reversible oxidation-reduction behaviour has been extensively used to control electron-transfer processes in electrochemical<ref>{{cite journal | vauthors = Sirbu D, Turta C, Gibson EA, Benniston AC | title = The ferrocene effect: enhanced electrocatalytic hydrogen production using meso-tetraferrocenyl porphyrin palladium(II) and copper(II) complexes | journal = Dalton Transactions | volume = 44 | issue = 33 | pages = 14646–14655 | date = September 2015 | pmid = 26213204 | doi = 10.1039/C5DT02191J | url = https://eprint.ncl.ac.uk/fulltext.aspx?url=214473/2DD72927-25BC-4809-8636-5FDB014D7CB0.pdf&pub_id=214473 }}</ref><ref>{{cite journal | vauthors = Lennox AJ, Nutting JE, Stahl SS | title = Selective electrochemical generation of benzylic radicals enabled by ferrocene-based electron-transfer mediators | journal = Chemical Science | volume = 9 | issue = 2 | pages = 356–361 | date = January 2018 | pmid = 29732109 | pmc = 5909123 | doi = 10.1039/C7SC04032F | doi-access = free }}</ref> and photochemical<ref>{{Cite journal| vauthors = Dannenberg JJ, Richards JH |date=1965-04-01|title=Photosensitization by Ferrocene. Photochemistry of Higher Electronic Excited States|journal=Journal of the American Chemical Society|volume=87|issue=7|pages=1626–1627|doi=10.1021/ja01085a048|issn=0002-7863}}</ref><ref>{{Cite journal| vauthors = Sirbu D, Turta C, Benniston AC, Abou-Chahine F, Lemmetyinen H, Tkachenko NV, Wood C, Gibson E | display-authors = 6 |date=2014-05-23|title=Synthesis and properties of a meso- tris–ferrocene appended zinc(II) porphyrin and a critical evaluation of its dye sensitised solar cell (DSSC) performance |journal=RSC Advances|language=en|volume=4|issue=43|pages=22733–22742|doi=10.1039/C4RA03105A|bibcode=2014RSCAd...422733S |issn=2046-2069| url = https://eprint.ncl.ac.uk/fulltext.aspx?url=199647/CED25CA5-111E-4500-8188-DCFC5D4D7190.pdf&pub_id=199647}}</ref> systems. |
||
[[File:Biferrocene.svg|thumb|left| |
[[File:Biferrocene.svg|thumb|left|132px|The one-electron oxidized derivative of [[biferrocene]] has attracted much research attention.]] |
||
Substituents on the cyclopentadienyl ligands alters the redox potential in the expected way: electron-withdrawing groups such as a [[carboxylic acid]] shift the potential in the [[anodic]] direction (''i.e.'' made more positive), whereas electron-releasing groups such as [[methyl]] groups shift the potential in the [[Cathode|cathodic]] direction (more negative). Thus, [[decamethylferrocene]] is much more easily oxidised than ferrocene and can even be oxidised to the corresponding dication.<ref>{{cite journal | vauthors = Malischewski M, Adelhardt M, Sutter J, Meyer K, Seppelt K | title = Isolation and structural and electronic characterization of salts of the decamethylferrocene dication | journal = Science | volume = 353 | issue = 6300 | pages = 678–682 | date = August 2016 | pmid = 27516596 | doi = 10.1126/science.aaf6362 | s2cid = 43385610 | bibcode = 2016Sci...353..678M }}</ref> Ferrocene is often used as an [[internal standard]] for calibrating redox potentials in non-aqueous [[electrochemistry]]. |
Substituents on the cyclopentadienyl ligands alters the redox potential in the expected way: electron-withdrawing groups such as a [[carboxylic acid]] shift the potential in the [[anodic]] direction (''i.e.'' made more positive), whereas electron-releasing groups such as [[methyl]] groups shift the potential in the [[Cathode|cathodic]] direction (more negative). Thus, [[decamethylferrocene]] is much more easily oxidised than ferrocene and can even be oxidised to the corresponding dication.<ref>{{cite journal | vauthors = Malischewski M, Adelhardt M, Sutter J, Meyer K, Seppelt K | title = Isolation and structural and electronic characterization of salts of the decamethylferrocene dication | journal = Science | volume = 353 | issue = 6300 | pages = 678–682 | date = August 2016 | pmid = 27516596 | doi = 10.1126/science.aaf6362 | s2cid = 43385610 | bibcode = 2016Sci...353..678M }}</ref> Ferrocene is often used as an [[internal standard]] for calibrating redox potentials in non-aqueous [[electrochemistry]]. |
||
| Line 186: | Line 166: | ||
===Fuel additives=== |
===Fuel additives=== |
||
Ferrocene and its derivatives are [[antiknock agent]]s used in the fuel for [[petrol engine]]s. They are safer than previously used [[tetraethyllead]].<ref>{{cite conference | vauthors = Bennett J | conference = International Conference on Automotive Technology | location = Istanbul | date = 26 November 2004 |url=http://www.osd.org.tr/14.pdf |title=Application of fuel additives |url-status=dead |archive-url=https://web.archive.org/web/20060505193757/http://www.osd.org.tr/14.pdf |archive-date=2006-05-05 }}</ref> Petrol additive solutions containing ferrocene can be added to unleaded petrol to enable its use in vintage cars designed to run on leaded petrol.<ref>{{cite patent|country=US|number=4104036|title=Iron-containing motor fuel compositions and method for using same |inventor = Chao TS | assign1 = Atlantic Richfield Co |pubdate= 1978-08-01}}</ref> The [[iron]]-containing deposits formed from ferrocene can form a [[conductive]] coating on [[spark plug]] surfaces. Ferrocene polyglycol copolymers, prepared by effecting a polycondensation reaction between a ferrocene derivative and a substituted dihydroxy alcohol, has promise as a component of rocket propellants. These copolymers provide rocket propellants with heat stability, serving as a propellant binder and controlling propellant burn rate.<ref>{{cite patent | inventor = Dewey FM | assign1 = U.S. Air Force | title = Ferrocene Polyglycols | country = US | number = 3598850 | fdate = June 11, 1969 | gdate = Aug. 10, 1971 | url = https://patentimages.storage.googleapis.com/6f/2a/1c/dad6147ea46bcb/US3598850.pdf | postscript = . |
Ferrocene and its derivatives are [[antiknock agent]]s used in the fuel for [[petrol engine]]s. They are safer than previously used [[tetraethyllead]].<ref>{{cite conference | vauthors = Bennett J | conference = International Conference on Automotive Technology | location = Istanbul | date = 26 November 2004 |url=http://www.osd.org.tr/14.pdf |title=Application of fuel additives |url-status=dead |archive-url=https://web.archive.org/web/20060505193757/http://www.osd.org.tr/14.pdf |archive-date=2006-05-05 }}</ref> Petrol additive solutions containing ferrocene can be added to unleaded petrol to enable its use in vintage cars designed to run on leaded petrol.<ref>{{cite patent|country=US|number=4104036|title=Iron-containing motor fuel compositions and method for using same |inventor = Chao TS | assign1 = Atlantic Richfield Co |pubdate= 1978-08-01}}</ref> The [[iron]]-containing deposits formed from ferrocene can form a [[conductive]] coating on [[spark plug]] surfaces. Ferrocene polyglycol copolymers, prepared by effecting a polycondensation reaction between a ferrocene derivative and a substituted dihydroxy alcohol, has promise as a component of rocket propellants. These copolymers provide rocket propellants with heat stability, serving as a propellant binder and controlling propellant burn rate.<ref>{{cite patent | inventor = Dewey FM | assign1 = U.S. Air Force | title = Ferrocene Polyglycols | country = US | number = 3598850 | fdate = June 11, 1969 | gdate = Aug. 10, 1971 | url = https://patentimages.storage.googleapis.com/6f/2a/1c/dad6147ea46bcb/US3598850.pdf | postscript = .}}</ref> |
||
Ferrocene has been found to be effective at reducing smoke and sulfur trioxide produced when burning coal. The addition by any practical means, impregnating the coal or adding ferrocene to the combustion chamber, can significantly reduce the amount of these undesirable byproducts, even with a small amount of the metal cyclopentadienyl compound.<ref>{{cite patent | inventor = Kerley RV | assign1 = Ethyl Corporation | title = Coal Combustion Process and Composition | country = US | number = 3927992 | fdate = November 23, 1971 | gdate = December 23, 1975 | url = https://patentimages.storage.googleapis.com/0d/03/57/c94e635d15e1fb/US3927992.pdf | postscript = . }}</ref> |
Ferrocene has been found to be effective at reducing smoke and sulfur trioxide produced when burning coal. The addition by any practical means, impregnating the coal or adding ferrocene to the combustion chamber, can significantly reduce the amount of these undesirable byproducts, even with a small amount of the metal cyclopentadienyl compound.<ref>{{cite patent | inventor = Kerley RV | assign1 = Ethyl Corporation | title = Coal Combustion Process and Composition | country = US | number = 3927992 | fdate = November 23, 1971 | gdate = December 23, 1975 | url = https://patentimages.storage.googleapis.com/0d/03/57/c94e635d15e1fb/US3927992.pdf | postscript = . }}</ref> |
||
===Pharmaceuticals=== |
===Pharmaceuticals=== |
||
[[File:Ferroceron.svg|thumb|Ferrocerone]] |
|||
Ferrocene derivatives have been investigated as drugs,<ref>{{cite journal | vauthors = van Staveren DR, Metzler-Nolte N | title = Bioorganometallic chemistry of ferrocene | journal = Chemical Reviews | volume = 104 | issue = 12 | pages = 5931–5985 | date = December 2004 | pmid = 15584693 | doi = 10.1021/cr0101510 }}</ref> with one compound [[ferrocerone]] approved for use in the USSR in the 1970s, though it is no longer marketed today.<ref>{{cite journal | vauthors = Ong YC, Gasser G | title = Organometallic compounds in drug discovery: Past, present and future | journal = Drug Discovery Today: Technologies | volume = 37 | pages = 117–124 | date = December 2020 | pmid = 34895650 | doi = 10.1016/j.ddtec.2019.06.001 | s2cid = 198268304 | url = https://hal.archives-ouvertes.fr/hal-02169801/file/Organometallic%20Compounds%20in%20Drug%20Discovery%20Past%2C%20Present%20and%20futur.pdf }}</ref> Only one drug has entered clinical trials in recent years, [[Ferroquine]] (7-chloro-N-(2-((dimethylamino)methyl)ferrocenyl)quinolin-4-amine), an [[antimalarial]],<ref name="BiotNosten2011">{{cite journal | vauthors = Biot C, Nosten F, Fraisse L, Ter-Minassian D, Khalife J, Dive D | title = The antimalarial ferroquine: from bench to clinic | journal = Parasite | volume = 18 | issue = 3 | pages = 207–214 | date = August 2011 | pmid = 21894260 | pmc = 3671469 | doi = 10.1051/parasite/2011183207 }} {{open access}}</ref><ref>{{cite journal | vauthors = Roux C, Biot C | title = Ferrocene-based antimalarials | journal = Future Medicinal Chemistry | volume = 4 | issue = 6 | pages = 783–797 | date = April 2012 | pmid = 22530641 | doi = 10.4155/fmc.12.26 }}</ref><ref>{{cite journal | vauthors = Wani WA, Jameel E, Baig U, Mumtazuddin S, Hun LT | title = Ferroquine and its derivatives: new generation of antimalarial agents | journal = European Journal of Medicinal Chemistry | volume = 101 | pages = 534–551 | date = August 2015 | pmid = 26188909 | doi = 10.1016/j.ejmech.2015.07.009 | pmc = 7115395 }}</ref> which has reached Phase IIb trials.<ref>{{cite journal | vauthors = Adoke Y, Zoleko-Manego R, Ouoba S, Tiono AB, Kaguthi G, Bonzela JE, Duong TT, Nahum A, Bouyou-Akotet M, Ogutu B, Ouedraogo A, Macintyre F, Jessel A, Laurijssens B, Cherkaoui-Rbati MH, Cantalloube C, Marrast AC, Bejuit R, White D, Wells TN, Wartha F, Leroy D, Kibuuka A, Mombo-Ngoma G, Ouattara D, Mugenya I, Phuc BQ, Bohissou F, Mawili-Mboumba DP, Olewe F, Soulama I, Tinto H | display-authors = 6 | title = A randomized, double-blind, phase 2b study to investigate the efficacy, safety, tolerability and pharmacokinetics of a single-dose regimen of ferroquine with artefenomel in adults and children with uncomplicated Plasmodium falciparum malaria | journal = Malaria Journal | volume = 20 | issue = 1 | pages = 222 | date = May 2021 | pmid = 34011358 | doi = 10.1186/s12936-021-03749-4 | pmc = 8135182 }}</ref> Ferrocene-containing polymer-based drug delivery systems have been investigated.<ref>{{Cite journal| vauthors = Gu H, Mu S, Qiu G, Liu X, Zhang L, Yuan Y, Astruc D |date=June 2018|title=Redox-stimuli-responsive drug delivery systems with supramolecular ferrocenyl-containing polymers for controlled release|journal=Coordination Chemistry Reviews|volume=364|pages=51–85|doi=10.1016/j.ccr.2018.03.013|s2cid=103022297 |issn=0010-8545}}</ref> |
|||
Ferrocene derivatives have been investigated as drugs,<ref>{{cite journal | vauthors = van Staveren DR, Metzler-Nolte N | title = Bioorganometallic chemistry of ferrocene | journal = Chemical Reviews | volume = 104 | issue = 12 | pages = 5931–5985 | date = December 2004 | pmid = 15584693 | doi = 10.1021/cr0101510 }}</ref> with one compound {{ill|ferrocerone|ru|Ферроцерон}} approved for use in the USSR in the 1970s as an [[iron supplement]], though it is no longer marketed today.<ref>{{cite journal | vauthors = Ong YC, Gasser G | title = Organometallic compounds in drug discovery: Past, present and future | journal = Drug Discovery Today: Technologies | volume = 37 | pages = 117–124 | date = December 2020 | pmid = 34895650 | doi = 10.1016/j.ddtec.2019.06.001 | s2cid = 198268304 | url = https://hal.archives-ouvertes.fr/hal-02169801/file/Organometallic%20Compounds%20in%20Drug%20Discovery%20Past%2C%20Present%20and%20futur.pdf }}</ref> Only one drug has entered clinical trials in recent years, [[Ferroquine]] (7-chloro-N-(2-((dimethylamino)methyl)ferrocenyl)quinolin-4-amine), an [[antimalarial]],<ref name="BiotNosten2011">{{cite journal | vauthors = Biot C, Nosten F, Fraisse L, Ter-Minassian D, Khalife J, Dive D | title = The antimalarial ferroquine: from bench to clinic | journal = Parasite | volume = 18 | issue = 3 | pages = 207–214 | date = August 2011 | pmid = 21894260 | pmc = 3671469 | doi = 10.1051/parasite/2011183207 }} {{open access}}</ref><ref>{{cite journal | vauthors = Roux C, Biot C | title = Ferrocene-based antimalarials | journal = Future Medicinal Chemistry | volume = 4 | issue = 6 | pages = 783–797 | date = April 2012 | pmid = 22530641 | doi = 10.4155/fmc.12.26 }}</ref><ref>{{cite journal | vauthors = Wani WA, Jameel E, Baig U, Mumtazuddin S, Hun LT | title = Ferroquine and its derivatives: new generation of antimalarial agents | journal = European Journal of Medicinal Chemistry | volume = 101 | pages = 534–551 | date = August 2015 | pmid = 26188909 | doi = 10.1016/j.ejmech.2015.07.009 | pmc = 7115395 }}</ref> which has reached Phase IIb trials.<ref>{{cite journal | vauthors = Adoke Y, Zoleko-Manego R, Ouoba S, Tiono AB, Kaguthi G, Bonzela JE, Duong TT, Nahum A, Bouyou-Akotet M, Ogutu B, Ouedraogo A, Macintyre F, Jessel A, Laurijssens B, Cherkaoui-Rbati MH, Cantalloube C, Marrast AC, Bejuit R, White D, Wells TN, Wartha F, Leroy D, Kibuuka A, Mombo-Ngoma G, Ouattara D, Mugenya I, Phuc BQ, Bohissou F, Mawili-Mboumba DP, Olewe F, Soulama I, Tinto H | display-authors = 6 | title = A randomized, double-blind, phase 2b study to investigate the efficacy, safety, tolerability and pharmacokinetics of a single-dose regimen of ferroquine with artefenomel in adults and children with uncomplicated Plasmodium falciparum malaria | journal = Malaria Journal | volume = 20 | issue = 1 | pages = 222 | date = May 2021 | pmid = 34011358 | doi = 10.1186/s12936-021-03749-4 | pmc = 8135182 | doi-access = free }}</ref> Ferrocene-containing polymer-based drug delivery systems have been investigated.<ref>{{Cite journal| vauthors = Gu H, Mu S, Qiu G, Liu X, Zhang L, Yuan Y, Astruc D |date=June 2018|title=Redox-stimuli-responsive drug delivery systems with supramolecular ferrocenyl-containing polymers for controlled release|journal=Coordination Chemistry Reviews|volume=364|pages=51–85|doi=10.1016/j.ccr.2018.03.013|s2cid=103022297 |issn=0010-8545}}</ref> |
|||
[[Image:Ferroquine.png|thumb|220 px|Ferroquine]] |
[[Image:Ferroquine.png|thumb|220 px|Ferroquine]] |
||
The anticancer activity of ferrocene derivatives was first investigated in the late 1970s, when derivatives bearing [[amine]] or [[amide]] groups were tested against lymphocytic [[leukemia]].<ref name=":0">{{Cite journal| vauthors = Ornelas C |s2cid=56521492|title=Application of ferrocene and its derivatives in cancer research|journal=New Journal of Chemistry|volume=35|issue=10|pages=1973|doi=10.1039/c1nj20172g|year=2011}}</ref> Some ferrocenium salts exhibit anticancer activity, but no compound has seen evaluation in the clinic.<ref name=Babin>{{cite journal | vauthors = Babin VN, Belousov YA, Borisov VI, Gumenyuk VV, Nekrasov YS, Ostrovskaya LA, Sviridova IK, Sergeeva NS, Simenel AA, Snegur LV | display-authors = 6 | year = 2014 | title = Ferrocenes as potential anticancer drugs. Facts and hypotheses | journal = Russ. Chem. Bull. | volume = 63 | issue = 11| pages = 2405–2422 | doi = 10.1007/s11172-014-0756-7 | s2cid = 94618726 }}</ref> |
The anticancer activity of ferrocene derivatives was first investigated in the late 1970s, when derivatives bearing [[amine]] or [[amide]] groups were tested against lymphocytic [[leukemia]].<ref name=":0">{{Cite journal| vauthors = Ornelas C |s2cid=56521492|title=Application of ferrocene and its derivatives in cancer research|journal=New Journal of Chemistry|volume=35|issue=10|pages=1973|doi=10.1039/c1nj20172g|year=2011}}</ref> Some ferrocenium salts exhibit anticancer activity, but no compound has seen evaluation in the clinic.<ref name=Babin>{{cite journal | vauthors = Babin VN, Belousov YA, Borisov VI, Gumenyuk VV, Nekrasov YS, Ostrovskaya LA, Sviridova IK, Sergeeva NS, Simenel AA, Snegur LV | display-authors = 6 | year = 2014 | title = Ferrocenes as potential anticancer drugs. Facts and hypotheses | journal = Russ. Chem. Bull. | volume = 63 | issue = 11| pages = 2405–2422 | doi = 10.1007/s11172-014-0756-7 | s2cid = 94618726 }}</ref> Ferrocene derivatives have strong inhibitory activity against human lung cancer cell line A549, colorectal cancer cell line HCT116, and breast cancer cell line MCF-7.<ref>{{cite patent | inventor = Yong J, Lu C | assign1 = Xiamen Institute of Rare Earth Materials | title = Ferrocene Derivative, Preparation Method and Use Thereof. | country = US | number = 9738673 | fdate = November 29, 2016 | gdate = August 22, 2017 | url = https://patentimages.storage.googleapis.com/dd/6e/d6/9fd8e3c5c96b67/US9738673.pdf | postscript = . }}</ref> An experimental drug was reported which is a ferrocenyl version of [[tamoxifen]].<ref name = top2003 /> The idea is that the tamoxifen will bind to the [[estrogen]] binding sites, resulting in cytotoxicity.<ref name=top2003>{{cite journal | vauthors = Top S, Vessières A, Leclercq G, Quivy J, Tang J, Vaissermann J, Huché M, Jaouen G | display-authors = 6 | title = Synthesis, biochemical properties and molecular modelling studies of organometallic specific estrogen receptor modulators (SERMs), the ferrocifens and hydroxyferrocifens: evidence for an antiproliferative effect of hydroxyferrocifens on both hormone-dependent and hormone-independent breast cancer cell lines | journal = Chemistry: A European Journal | volume = 9 | issue = 21 | pages = 5223–5236 | date = November 2003 | pmid = 14613131 | doi = 10.1002/chem.200305024 }}</ref><ref>{{cite journal|journal=[[Chemical and Engineering News]]|date=16 September 2002| title= The Bio Side of Organometallics| vauthors = Dagani R | volume = 80| issue= 37| pages = 23–29| url=http://pubs.acs.org/cen/science/8037/8037sci1.html|doi=10.1021/cen-v080n037.p023}}</ref> |
||
Ferrocifens are exploited for cancer applications by a French biotech, Feroscan, founded by Pr. Gerard Jaouen. |
Ferrocifens are exploited for cancer applications by a French biotech, Feroscan, founded by Pr. Gerard Jaouen. |
||
| Line 221: | Line 202: | ||
===Materials chemistry=== |
===Materials chemistry=== |
||
[[File:Wettability of a silica surface with a bound ferrocene-substituted polymer.jpg|left|thumb|500px|Strands of an uncharged ferrocene-substituted polymer are tethered to a [[hydrophobic]] [[silica]] surface. |
[[File:Wettability of a silica surface with a bound ferrocene-substituted polymer.jpg|left|thumb|500px|Strands of an uncharged ferrocene-substituted polymer are tethered to a [[hydrophobic]] [[silica]] surface. Oxidation of the ferrocenyl groups produces a [[hydrophilic]] surface due to electrostatic attractions between the resulting charges and the polar solvent.<ref name = Pietschnig />]] |
||
Ferrocene, a precursor to iron nanoparticles, can be used as a catalyst for the production of carbon nanotubes.<ref>{{cite journal| vauthors = Conroy D, Moisala A, Cardoso S, Windle A, Davidson J |journal=[[Chemical Engineering Science|Chem. Eng. Sci.]]|year=2010|volume=65|pages=2965–2977|doi=10.1016/j.ces.2010.01.019|title=Carbon nanotube reactor: Ferrocene decomposition, iron particle growth, nanotube aggregation and scale-up|issue=10}}</ref> |
Ferrocene, a precursor to iron nanoparticles, can be used as a catalyst for the production of carbon nanotubes.<ref>{{cite journal| vauthors = Conroy D, Moisala A, Cardoso S, Windle A, Davidson J |journal=[[Chemical Engineering Science|Chem. Eng. Sci.]]|year=2010|volume=65|pages=2965–2977|doi=10.1016/j.ces.2010.01.019|title=Carbon nanotube reactor: Ferrocene decomposition, iron particle growth, nanotube aggregation and scale-up|issue=10}}</ref> [[Vinylferrocene]] can be converted to (polyvinylferrocene, PVFc), a ferrocenyl version of [[polystyrene]] (the phenyl groups are replaced with ferrocenyl groups). Another [[Polyferrocenes|polyferrocene]] which can be formed is poly(2-(methacryloyloxy)ethyl ferrocenecarboxylate), PFcMA. In addition to using organic polymer backbones, these pendant ferrocene units have been attached to inorganic backbones such as [[polysiloxane]]s, [[polyphosphazene]]s, and poly[[phosphinoborane]]s, (–PH(R)–BH<sub>2</sub>–)<sub>''n''</sub>, and the resulting materials exhibit unusual physical and electronic properties relating to the ferrocene / ferrocinium redox couple.<ref name = Pietschnig>{{cite journal | vauthors = Pietschnig R | title = Polymers with pendant ferrocenes | journal = Chemical Society Reviews | volume = 45 | issue = 19 | pages = 5216–5231 | date = October 2016 | pmid = 27156979 | doi = 10.1039/C6CS00196C | doi-access = free }}</ref> Both PVFc and PFcMA have been tethered onto [[silica]] wafers and the [[wettability]] measured when the polymer chains are uncharged and when the ferrocene moieties are oxidised to produce positively charged groups. The [[contact angle]] with water on the PFcMA-coated wafers was 70° smaller following oxidation, while in the case of PVFc the decrease was 30°, and the switching of wettability is reversible. In the PFcMA case, the effect of lengthening the chains and hence introducing more ferrocene groups is significantly larger reductions in the contact angle upon oxidation.<ref name = Pietschnig /><ref>{{cite journal| vauthors = Elbert J, Gallei M, Rüttiger C, Brunsen A, Didzoleit H, Stühn B, Rehahn M |journal = [[Organometallics]]|year = 2013|volume = 32|issue = 20|pages = 5873–5878|title = Ferrocene Polymers for Switchable Surface Wettability|doi = 10.1021/om400468p}}</ref> |
||
== See also == |
== See also == |
||
| Line 229: | Line 210: | ||
== References == |
== References == |
||
<references> |
<references> |
||
<ref name="pauson">{{cite journal| vauthors = Kealy TJ, Pauson PL |author-link2 = Peter Pauson|title = A New Type of Organo-Iron Compound|journal = [[Nature (journal)|Nature]]|year = 1951|volume = 168|pages = 1039–1040|doi = 10.1038/1681039b0 |issue = 4285|bibcode = 1951Natur.168.1039K|s2cid = 4181383}}</ref> |
|||
<ref name="miller">{{cite journal| vauthors = Miller SA, Tebboth JA, Tremaine JF |journal= [[Journal of the Chemical Society|J. Chem. Soc.]]|year=1952| pages= 632–635| title=114. Dicyclopentadienyliron |doi=10.1039/JR9520000632}}</ref> |
|||
<!--Original papers--> |
|||
<ref name="wilk52">{{cite journal |author-link1=Geoffrey Wilkinson |author-link4=Robert Burns Woodward |vauthors=Wilkinson G, Rosenblum M, Whiting MC, Woodward RB |year=1952-03-24 |title=The structure of iron ''bis''-cyclopentadienyl |journal=[[Journal of the American Chemical Society|J. Am. Chem. Soc.]] |volume=74 |issue=8 |pages=2125–2126 |doi=10.1021/ja01128a527}}</ref> |
|||
<ref name= |
<ref name="fischer">{{cite journal | vauthors = Fischer EO, Pfab W |author-link1= Ernst Otto Fischer |title = Zur Kristallstruktur der Di-Cyclopentadienyl-Verbindungen des zweiwertigen Eisens, Kobalts und Nickels|trans-title=On the crystal structure of the bis-cyclopentadienyl compounds of divalent iron, cobalt and nickel |journal = Zeitschrift für Naturforschung B |year = 1952 |volume = 7 |issue = 7|pages = 377–379 |doi =10.1515/znb-1952-0701|doi-access = free}}</ref> |
||
<ref name="eiland52">{{Cite journal | vauthors = Eiland PF, Pepinsky R |year= 1952 |title= X-ray Examination of Iron Biscyclopentadienyl |journal= [[Journal of the American Chemical Society|J. Am. Chem. Soc.]] |volume= 74 |issue= 19 |page= 4971 |doi= 10.1021/ja01139a527}}</ref> |
|||
<ref name= |
<ref name="dunitz53">{{cite journal| vauthors = Dunitz JD, Orgel LE |title=Bis-Cyclopentadienyl – A Molecular Sandwich|journal= [[Nature (journal)|Nature]] |year=1953| volume=171 |pages= 121–122|doi = 10.1038/171121a0|issue=4342|bibcode = 1953Natur.171..121D |s2cid= 4263761}}</ref> |
||
<ref name="dunitz56">{{cite journal | vauthors = Dunitz JD, Orgel L, Rich A |title = The crystal structure of ferrocene |journal = [[Acta Crystallographica|Acta Crystallogr.]] |year = 1956 |volume = 9 |pages = 373–375 |doi = 10.1107/S0365110X56001091 |issue = 4|doi-access = free}}</ref> |
|||
<ref name= |
<ref name="haal68">{{cite journal | vauthors = Haaland A, Nilsson JE | year = 1968 | title = The Determination of Barriers to Internal Rotation by Means of Electron Diffraction. Ferrocene and Ruthenocene | journal = [[Acta Chemica Scandinavica|Acta Chem. Scand.]] | volume = 22 | pages = 2653–2670 | doi = 10.3891/acta.chem.scand.22-2653 | doi-access = free}}</ref> |
||
<ref name="seiler">{{Cite journal| vauthors = Seiler P, Dunitz JD |year=1982|title=Low-temperature crystallization of orthorhombic ferrocene: structure analysis at 98 K|journal=[[Acta Crystallographica Section B]]|volume=38|issue=6|pages=1741–1745|doi=10.1107/s0567740882007080|issn=0567-7408}}</ref> |
|||
<ref name= |
<ref name="coriani">{{cite journal | vauthors = Coriani S, Haaland A, Helgaker T, Jørgensen P | title = The equilibrium structure of ferrocene | journal = ChemPhysChem | volume = 7 | issue = 1 | pages = 245–249 | date = January 2006 | pmid = 16404766 | doi = 10.1002/cphc.200500339 }}</ref> |
||
<ref name="laszloRmon">{{cite journal | vauthors = Laszlo P, Hoffmann R | title = Ferrocene: Ironclad History or Rashomon Tale? | journal = Angewandte Chemie | volume = 39 | issue = 1 | pages = 123–124 | date = January 2000 | pmid = 10649350 | doi = 10.1002/(SICI)1521-3773(20000103)39:1<123::AID-ANIE123>3.0.CO;2-Z | author-link2 = Roald Hoffman}}</ref> |
|||
<ref name= |
<ref name="werner2012">{{cite journal | vauthors = Werner H | title = At least 60 years of ferrocene: the discovery and rediscovery of the sandwich complexes | journal = Angewandte Chemie | volume = 51 | issue = 25 | pages = 6052–6058 | date = June 2012 | pmid = 22573490 | doi = 10.1002/anie.201201598 }}</ref> |
||
<ref name="werner2008">{{cite book | vauthors = Werner H |year= 2008 |location= New York |title= Landmarks in Organo-Transition Metal Chemistry: A Personal View |publisher= Springer Science |pages= 161–63 |isbn= 978-0-387-09847-0 |url={{Google books|dP4LTfaPzAMC|page=PA161|keywords=|text=|plainurl=yes}}}}</ref> |
|||
<ref name="federman">{{Cite journal | vauthors = Federman Neto A, Pelegrino AC, Darin VA |year= 2004 |title= Ferrocene: 50 Years of Transition Metal Organometallic Chemistry — From Organic and Inorganic to Supramolecular Chemistry (Abstract) |journal= Trends in Organometallic Chemistry |volume= 4 |pages= 147–169 |url= http://www.researchtrends.net/tia/abstract.asp?in=0&vn=4&tid=9&aid=870&pub=2002&type=3 |publisher= [[Research Trends]]}}</ref> |
|||
<ref name=fischer2>{{cite journal | vauthors = Fischer EO, Pfab W |author-link1= Ernst Otto Fischer |title = Zur Kristallstruktur der Di-Cyclopentadienyl-Verbindungen des zweiwertigen Eisens, Kobalts und Nickels|trans-title=On the crystal structure of the bis-cyclopentadienyl compounds of divalent iron, cobalt and nickel |journal = Zeitschrift für Naturforschung B |year = 1952 |volume = 7 |issue = 7|pages = 377–379 |doi =10.1515/znb-1952-0701|doi-access = free }}</ref> |
|||
<ref name="mingos">{{cite journal | vauthors = Mingos DM |year= 2001 |title= A Historical Perspective on Dewar's Landmark Contribution to Organometallic Chemistry |journal= [[Journal of Organometallic Chemistry|J. Organomet. Chem.]] |volume= 635 |issue= 1–2 |pages= 1–8 |doi= 10.1016/S0022-328X(01)01155-X}}</ref> |
|||
<ref name= |
<ref name="mehr">{{cite book | vauthors = Mehrotra RC, Singh A |year= 2007 |title= Organometallic Chemistry: A Unified Approach |edition= 2nd |location= New Delhi |publisher= New Age International |pages= 261–67 |isbn= 978-81-224-1258-1 |url={{Google books|NSQy3mFKRM8C|page=PA262|keywords=|text=|plainurl=yes}}}}</ref> |
||
<ref name="okuda">{{Cite journal| vauthors = Okuda J |date=2016-12-28|title=Ferrocene - 65 Years After|journal=European Journal of Inorganic Chemistry|volume=2017|issue=2|pages=217–219|doi=10.1002/ejic.201601323|issn=1434-1948|doi-access=free}}</ref> |
|||
<ref name=dunitz53>{{cite journal| vauthors = Dunitz JD, Orgel LE |title=Bis-Cyclopentadienyl – A Molecular Sandwich|journal= [[Nature (journal)|Nature]] |year=1953| volume=171 |pages= 121–122|doi = 10.1038/171121a0|issue=4342|bibcode = 1953Natur.171..121D |s2cid= 4263761}}</ref> |
|||
<ref name=dunitz56>{{cite journal | vauthors = Dunitz JD, Orgel L, Rich A |title = The crystal structure of ferrocene |journal = [[Acta Crystallographica|Acta Crystallogr.]] |year = 1956 |volume = 9 |pages = 373–375 |doi = 10.1107/S0365110X56001091 |issue = 4|doi-access = free }}</ref> |
|||
<ref name=haal68>{{cite journal | vauthors = Haaland A, Nilsson JE | year = 1968 | title = The Determination of Barriers to Internal Rotation by Means of Electron Diffraction. Ferrocene and Ruthenocene | journal = [[Acta Chemica Scandinavica|Acta Chem. Scand.]] | volume = 22 | pages = 2653–2670 | doi = 10.3891/acta.chem.scand.22-2653 | doi-access = free }}</ref> |
|||
<ref name=seiler>{{Cite journal| vauthors = Seiler P, Dunitz JD |year=1982|title=Low-temperature crystallization of orthorhombic ferrocene: structure analysis at 98 K|journal=Acta Crystallographica Section B|volume=38|issue=6|pages=1741–1745|doi=10.1107/s0567740882007080|issn=0567-7408|doi-access=free}}</ref> |
|||
<ref name=coriani>{{cite journal | vauthors = Coriani S, Haaland A, Helgaker T, Jørgensen P | title = The equilibrium structure of ferrocene | journal = ChemPhysChem | volume = 7 | issue = 1 | pages = 245–249 | date = January 2006 | pmid = 16404766 | doi = 10.1002/cphc.200500339 }}</ref> |
|||
<!--Surveys and reviews--> |
|||
<ref name=hunt>{{cite journal | vauthors = Hunt LB |year= 1984 |title= The First Organometallic Compounds: William Christopher Zeise and his Platinum Complexes |journal= [[Platinum Metals Review|Platinum Metals Rev.]] |volume= 28 |issue= 2 |pages= 76–83 |url= http://www.platinummetalsreview.com/pdf/pmr-v28-i2-076-083.pdf}}</ref> |
|||
<ref name=laszloRmon>{{cite journal | vauthors = Laszlo P, Hoffmann R | title = Ferrocene: Ironclad History or Rashomon Tale? | journal = Angewandte Chemie | volume = 39 | issue = 1 | pages = 123–124 | date = January 2000 | pmid = 10649350 | doi = 10.1002/(SICI)1521-3773(20000103)39:1<123::AID-ANIE123>3.0.CO;2-Z | author-link2 = Roald Hoffman }}</ref> |
|||
<ref name=eisch2002>{{cite journal| vauthors = Eisch JJ |title=Henry Gilman: American Pioneer in the Rise of Organometallic Chemistry in Modern Science and Technology†|journal=Organometallics|volume=21|issue=25|year=2002|pages=5439–5463|issn=0276-7333|doi=10.1021/om0109408}}</ref> |
|||
<ref name=leigh>{{cite book | veditors = Leigh GJ, Winterton N |year= 2002|title= Modern Coordination Chemistry: The Legacy of Joseph Chatt |location= Cambridge, UK |publisher= RSC Publishing |pages= 101–10 |isbn= 978-0-85404-469-6 |url={{Google books|VoBxtPb5zCcC|page=PA101|keywords=|text=|plainurl=yes}}}}</ref> |
|||
<ref name=werner2012>{{cite journal | vauthors = Werner H | title = At least 60 years of ferrocene: the discovery and rediscovery of the sandwich complexes | journal = Angewandte Chemie | volume = 51 | issue = 25 | pages = 6052–6058 | date = June 2012 | pmid = 22573490 | doi = 10.1002/anie.201201598 }}</ref> |
|||
<ref name=werner2008>{{cite book | vauthors = Werner H |year= 2008 |location= New York |title= Landmarks in Organo-Transition Metal Chemistry: A Personal View |publisher= Springer Science |pages= 161–63 |isbn= 978-0-387-09847-0 |url={{Google books|dP4LTfaPzAMC|page=PA161|keywords=|text=|plainurl=yes}}}}</ref> |
|||
<ref name=federman>{{Cite journal | vauthors = Federman Neto A, Pelegrino AC, Darin VA |year= 2004 |title= Ferrocene: 50 Years of Transition Metal Organometallic Chemistry — From Organic and Inorganic to Supramolecular Chemistry (Abstract) |journal= Trends in Organometallic Chemistry |volume= 4 |pages= 147–169 |url= http://www.researchtrends.net/tia/abstract.asp?in=0&vn=4&tid=9&aid=870&pub=2002&type=3 |publisher= [[Research Trends]]}}</ref> |
|||
<ref name=mingos>{{cite journal | vauthors = Mingos DM |year= 2001 |title= A Historical Perspective on Dewar's Landmark Contribution to Organometallic Chemistry |journal= [[Journal of Organometallic Chemistry|J. Organomet. Chem.]] |volume= 635 |issue= 1–2 |pages= 1–8 |doi= 10.1016/S0022-328X(01)01155-X}}</ref> |
|||
<ref name=mehr>{{cite book | vauthors = Mehrotra RC, Singh A |year= 2007 |title= Organometallic Chemistry: A Unified Approach |edition= 2nd |location= New Delhi |publisher= New Age International |pages= 261–67 |isbn= 978-81-224-1258-1 |url={{Google books|NSQy3mFKRM8C|page=PA262|keywords=|text=|plainurl=yes}}}}</ref> |
|||
<ref name=okuda>{{Cite journal| vauthors = Okuda J |date=2016-12-28|title=Ferrocene - 65 Years After|journal=European Journal of Inorganic Chemistry|volume=2017|issue=2|pages=217–219|doi=10.1002/ejic.201601323|issn=1434-1948}}</ref> |
|||
</references> |
</references> |
||
Latest revision as of 17:00, 2 November 2024
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Ferrocene[1] | |||
Other names
| |||
| Identifiers | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.764 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H10Fe | |||
| Molar mass | 186.04 g/mol | ||
| Appearance | light orange powder | ||
| Odor | camphor-like | ||
| Density | 1.107 g/cm3 (0 °C) 1.490 g/cm3 (20 °C)[2] | ||
| Melting point | 172.5 °C (342.5 °F; 445.6 K)[4] | ||
| Boiling point | 249 °C (480 °F; 522 K) | ||
| Insoluble in water, soluble in most organic solvents | |||
| log P | 2.04050[3] | ||
| Structure | |||
| D5h (eclipsed) D5d (staggered) | |||
| Sandwich structure with iron centre | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 15 mg/m3 (total) TWA 5 mg/m3 (resp)[5] | ||
REL (Recommended)
|
TWA 10 mg/m3 (total) TWA 5 mg/m3 (resp)[5] | ||
IDLH (Immediate danger)
|
N.D.[5] | ||
| Related compounds | |||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Ferrocene is an organometallic compound with the formula Fe(C5H5)2. The molecule is a complex consisting of two cyclopentadienyl rings sandwiching a central iron atom. It is an orange solid with a camphor-like odor that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation Fe(C5H5)+2.[7] Ferrocene and the ferrocenium cation are sometimes abbreviated as Fc and Fc+ respectively.
The first reported synthesis of ferrocene was in 1951. Its unusual stability puzzled chemists, and required the development of new theory to explain its formation and bonding. The discovery of ferrocene and its many analogues, known as metallocenes, sparked excitement and led to a rapid growth in the discipline of organometallic chemistry. Geoffrey Wilkinson and Ernst Otto Fischer, both of whom worked on elucidating the structure of ferrocene, later shared the 1973 Nobel Prize in Chemistry for their work on organometallic sandwich compounds. Ferrocene itself has no large-scale applications, but has found more niche uses in catalysis, as a fuel additive, and as a tool in undergraduate education.
History
[edit]Discovery
[edit]Ferrocene was discovered by accident twice. The first known synthesis may have been made in the late 1940s by unknown researchers at Union Carbide, who tried to pass hot cyclopentadiene vapor through an iron pipe. The vapor reacted with the pipe wall, creating a "yellow sludge" that clogged the pipe. Years later, a sample of the sludge that had been saved was obtained and analyzed by Eugene O. Brimm, shortly after reading Kealy and Pauson's article, and was found to consist of ferrocene.[7][8]
The second time was around 1950, when Samuel A. Miller, John A. Tebboth, and John F. Tremaine, researchers at British Oxygen, were attempting to synthesize amines from hydrocarbons and nitrogen in a modification of the Haber process. When they tried to react cyclopentadiene with nitrogen at 300 °C, at atmospheric pressure, they were disappointed to see the hydrocarbon react with some source of iron, yielding ferrocene. While they too observed its remarkable stability, they put the observation aside and did not publish it until after Pauson reported his findings.[7][9][10] Kealy and Pauson were later provided with a sample by Miller et al., who confirmed that the products were the same compound.[8]
In 1951, Peter L. Pauson and Thomas J. Kealy at Duquesne University attempted to prepare fulvalene ((C5H4)2) by oxidative dimerization of cyclopentadiene (C5H6). To that end, they reacted the Grignard compound cyclopentadienyl magnesium bromide in diethyl ether with ferric chloride as an oxidizer.[7] However, instead of the expected fulvalene, they obtained a light orange powder of "remarkable stability", with the formula C10H10Fe.[8][11]
Determining the structure
[edit]
Pauson and Kealy conjectured that the compound had two cyclopentadienyl groups, each with a single covalent bond from the saturated carbon atom to the iron atom.[7] However, that structure was inconsistent with then-existing bonding models and did not explain the unexpected stability of the compound, and chemists struggled to find the correct structure.[10][12]
The structure was deduced and reported independently by three groups in 1952.[13] Robert Burns Woodward, Geoffrey Wilkinson, et al. deduced observe that the compound was diamagnetic and nonpolar.[14] A few months later they described its reactions as being typical of aromatic compounds such as benzene.[15] The name ferrocene was coined by Mark Whiting, a postdoc with Woodward.[16]. Ernst Otto Fischer and Wolfgang Pfab also noted ferrocene's diamagneticity and high symmetry. They also synthesize nickelocene and cobaltocene and confirmed they had the same structure.[17] Fischer described the structure as Doppelkegelstruktur ("double-cone structure"), although the term "sandwich" came to be preferred by British and American chemists.[18] Philip Frank Eiland and Raymond Pepinsky confirmed the structure through X-ray crystallography and later by NMR spectroscopy.[10][19][20][21]
The "sandwich" structure of ferrocene was shockingly novel and led to intensive theoretical studies. Application of molecular orbital theory with the assumption of a Fe2+ centre between two cyclopentadienide anions C5H−5 resulted in the successful Dewar–Chatt–Duncanson model, allowing correct prediction of the geometry of the molecule as well as explaining its remarkable stability.[22][23]
Impact
[edit]The discovery of ferrocene was considered so significant that Wilkinson and Fischer shared the 1973 Nobel Prize in Chemistry "for their pioneering work, performed independently, on the chemistry of the organometallic, called sandwich compounds".[24]
Structure and bonding
[edit]Mössbauer spectroscopy indicates that the iron center in ferrocene should be assigned the +2 oxidation state. Each cyclopentadienyl (Cp) ring should then be allocated a single negative charge. Thus ferrocene could be described as iron(II) bis(cyclopentadienide), Fe2+[C5H−5]2.
Each ring has six π-electrons, which makes them aromatic according to Hückel's rule. These π-electrons are then shared with the metal via covalent bonding. Since Fe2+ has six d-electrons, the complex attains an 18-electron configuration, which accounts for its stability. In modern notation, this sandwich structural model of the ferrocene molecule is denoted as Fe(η5-C5H5)2, where η denotes hapticity, the number of atoms through which each ring binds.
The carbon–carbon bond distances around each five-membered ring are all 1.40 Å, and all Fe–C bond distances are 2.04 Å. From room temperature down to 164 K, X-ray crystallography yields the monoclinic space group; the cyclopentadienide rings are a staggered conformation, resulting in a centrosymmetric molecule, with symmetry group D5d.[19] However, below 110 K, ferrocene crystallizes in an orthorhombic crystal lattice in which the Cp rings are ordered and eclipsed, so that the molecule has symmetry group D5h.[25] In the gas phase, electron diffraction[26] and computational studies[27] show that the Cp rings are eclipsed. While ferrocene has no permanent dipole moment at room temperature, between 172.8 and 163.5 K the molecule exhibits an "incommensurate modulation", breaking the D5 symmetry and acquiring an electric dipole.[28]
The Cp rings rotate with a low barrier about the Cp(centroid)–Fe–Cp(centroid) axis, as observed by measurements on substituted derivatives of ferrocene using 1H and 13C nuclear magnetic resonance spectroscopy. For example, methylferrocene (CH3C5H4FeC5H5) exhibits a singlet for the C5H5 ring.[29]
In solution, and at room temperature, eclipsed D5h ferrocene was determined to dominate over the staggered D5d conformer, as suggested by both Fourier-transform infrared spectroscopy and DFT calculations.[30]
Synthesis
[edit]Early methods
[edit]The first reported syntheses of ferrocene were nearly simultaneous. Pauson and Kealy synthesised ferrocene using iron(III) chloride and cyclopentadienyl magnesium bromide.[11] A redox reaction produces iron(II) chloride. The formation of fulvalene, the intended outcome does not occur.[8]

Another early synthesis of ferrocene was by Miller et al.,[9] who treated metallic iron with gaseous cyclopentadiene at elevated temperature.[31] An approach using iron pentacarbonyl was also reported.[32]
- Fe(CO)5 + 2 C5H6 → Fe(C5H5)2 + 5 CO + H2
Via alkali cyclopentadienide
[edit]More efficient preparative methods are generally a modification of the original transmetalation sequence using either commercially available sodium cyclopentadienide[33] or freshly cracked cyclopentadiene deprotonated with potassium hydroxide[34] and reacted with anhydrous iron(II) chloride in ethereal solvents.
Modern modifications of Pauson and Kealy's original Grignard approach are known:
- Using sodium cyclopentadienide: 2 NaC5H5 + FeCl2 → Fe(C5H5)2 + 2 NaCl
- Using freshly-cracked cyclopentadiene: FeCl2·4H2O + 2 C5H6 + 2 KOH → Fe(C5H5)2 + 2 KCl + 6 H2O
- Using an iron(II) salt with a Grignard reagent: 2 C5H5MgBr + FeCl2 → Fe(C5H5)2 + 2 MgBrCl
Even some amine bases (such as diethylamine) can be used for the deprotonation, though the reaction proceeds more slowly than when using stronger bases:[33]
- 2 C5H6 + 2 (CH3CH2)2NH + FeCl2 → Fe(C5H5)2 + 2 (CH3CH2)2NH2Cl
Direct transmetalation can also be used to prepare ferrocene from some other metallocenes, such as manganocene:[35]
- FeCl2 + Mn(C5H5)2 → MnCl2 + Fe(C5H5)2
Properties
[edit]
Ferrocene is an air-stable orange solid with a camphor-like odor. As expected for a symmetric, uncharged species, ferrocene is soluble in normal organic solvents, such as benzene, but is insoluble in water. It is stable to temperatures as high as 400 °C.[36]
Ferrocene readily sublimes, especially upon heating in a vacuum. Its vapor pressure is about 1 Pa at 25 °C, 10 Pa at 50 °C, 100 Pa at 80 °C, 1000 Pa at 116 °C, and 10,000 Pa (nearly 0.1 atm) at 162 °C.[37][38]
Reactions
[edit]With electrophiles
[edit]Ferrocene undergoes many reactions characteristic of aromatic compounds, enabling the preparation of substituted derivatives. A common undergraduate experiment is the Friedel–Crafts reaction of ferrocene with acetic anhydride (or acetyl chloride) in the presence of phosphoric acid as a catalyst. Under conditions for a Mannich reaction, ferrocene gives N,N-dimethylaminomethylferrocene.
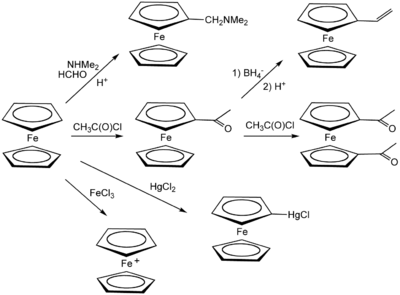
Ferrocene can itself be oxidized to the ferrocenium cation (Fc+); the ferrocene/ferrocenium couple is often used as a reference in electrochemistry.[12]
It is an aromatic substance and undergoes substitution reactions rather than addition reactions on the cyclopentadienyl ligands. For example, Friedel-Crafts acylation of ferrocene with acetic anhydride yields acetylferrocene[39] just as acylation of benzene yields acetophenone under similar conditions. Vilsmeier-Haack reaction (formylation) using formylanilide and phosphorus oxychloride gives ferrocenecarboxaldehyde. Diformylation does not occur readily, showing the electronic communication between the two rings.[40]
Protonation of ferrocene allows isolation of [Cp2FeH]PF6.[41]
In the presence of aluminium chloride, Me2NPCl2 and ferrocene react to give ferrocenyl dichlorophosphine,[42] whereas treatment with phenyldichlorophosphine under similar conditions forms P,P-diferrocenyl-P-phenyl phosphine.[43]
Ferrocene reacts with P4S10 forms a diferrocenyl-dithiadiphosphetane disulfide.[44]
Lithiation
[edit]Ferrocene reacts with butyllithium to give 1,1′-dilithioferrocene, which is a versatile nucleophile. In combination with butyllithiium, tert-butyllithium produces monolithioferrocene.[45]
Redox chemistry
[edit]Ferrocene undergoes a one-electron oxidation at around 0.4 V versus a saturated calomel electrode (SCE), becoming ferrocenium. This reversible oxidation has been used as standard in electrochemistry as Fc+/Fc = 0.64 V versus the standard hydrogen electrode,[46] however other values have been reported.[47] Ferrocenium tetrafluoroborate is a common reagent.[48] The remarkably reversible oxidation-reduction behaviour has been extensively used to control electron-transfer processes in electrochemical[49][50] and photochemical[51][52] systems.
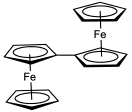
Substituents on the cyclopentadienyl ligands alters the redox potential in the expected way: electron-withdrawing groups such as a carboxylic acid shift the potential in the anodic direction (i.e. made more positive), whereas electron-releasing groups such as methyl groups shift the potential in the cathodic direction (more negative). Thus, decamethylferrocene is much more easily oxidised than ferrocene and can even be oxidised to the corresponding dication.[53] Ferrocene is often used as an internal standard for calibrating redox potentials in non-aqueous electrochemistry.
Stereochemistry of substituted ferrocenes
[edit]
Disubstituted ferrocenes can exist as either 1,2-, 1,3- or 1,1′- isomers, none of which are interconvertible. Ferrocenes that are asymmetrically disubstituted on one ring are chiral – for example [CpFe(EtC5H3Me)]. This planar chirality arises despite no single atom being a stereogenic centre. The substituted ferrocene shown at right (a 4-(dimethylamino)pyridine derivative) has been shown to be effective when used for the kinetic resolution of racemic secondary alcohols.[54] Several approaches have been developed to asymmetrically 1,1′-functionalise the ferrocene.[55]
Applications of ferrocene and its derivatives
[edit]Ferrocene and its numerous derivatives have no large-scale applications, but have many niche uses that exploit the unusual structure (ligand scaffolds, pharmaceutical candidates), robustness (anti-knock formulations, precursors to materials), and redox (reagents and redox standards).
Ligand scaffolds
[edit]Chiral ferrocenyl phosphines are employed as ligands for transition-metal catalyzed reactions. Some of them have found industrial applications in the synthesis of pharmaceuticals and agrochemicals. For example, the diphosphine 1,1′-bis(diphenylphosphino)ferrocene (dppf) is a valued ligand for palladium-coupling reactions and Josiphos ligand is useful for hydrogenation catalysis.[56] They are named after the technician who made the first one, Josi Puleo.[57][58]

Fuel additives
[edit]Ferrocene and its derivatives are antiknock agents used in the fuel for petrol engines. They are safer than previously used tetraethyllead.[59] Petrol additive solutions containing ferrocene can be added to unleaded petrol to enable its use in vintage cars designed to run on leaded petrol.[60] The iron-containing deposits formed from ferrocene can form a conductive coating on spark plug surfaces. Ferrocene polyglycol copolymers, prepared by effecting a polycondensation reaction between a ferrocene derivative and a substituted dihydroxy alcohol, has promise as a component of rocket propellants. These copolymers provide rocket propellants with heat stability, serving as a propellant binder and controlling propellant burn rate.[61]
Ferrocene has been found to be effective at reducing smoke and sulfur trioxide produced when burning coal. The addition by any practical means, impregnating the coal or adding ferrocene to the combustion chamber, can significantly reduce the amount of these undesirable byproducts, even with a small amount of the metal cyclopentadienyl compound.[62]
Pharmaceuticals
[edit]
Ferrocene derivatives have been investigated as drugs,[63] with one compound ferrocerone approved for use in the USSR in the 1970s as an iron supplement, though it is no longer marketed today.[64] Only one drug has entered clinical trials in recent years, Ferroquine (7-chloro-N-(2-((dimethylamino)methyl)ferrocenyl)quinolin-4-amine), an antimalarial,[65][66][67] which has reached Phase IIb trials.[68] Ferrocene-containing polymer-based drug delivery systems have been investigated.[69]

The anticancer activity of ferrocene derivatives was first investigated in the late 1970s, when derivatives bearing amine or amide groups were tested against lymphocytic leukemia.[70] Some ferrocenium salts exhibit anticancer activity, but no compound has seen evaluation in the clinic.[71] Ferrocene derivatives have strong inhibitory activity against human lung cancer cell line A549, colorectal cancer cell line HCT116, and breast cancer cell line MCF-7.[72] An experimental drug was reported which is a ferrocenyl version of tamoxifen.[73] The idea is that the tamoxifen will bind to the estrogen binding sites, resulting in cytotoxicity.[73][74]
Ferrocifens are exploited for cancer applications by a French biotech, Feroscan, founded by Pr. Gerard Jaouen.
Solid rocket propellant
[edit]Ferrocene and related derivatives are used as powerful burn rate catalysts in ammonium perchlorate composite propellant.[75]
Derivatives and variations
[edit]Ferrocene analogues can be prepared with variants of cyclopentadienyl. For example, bisindenyliron and bisfluorenyliron.[58]

Carbon atoms can be replaced by heteroatoms as illustrated by Fe(η5-C5Me5)(η5-P5) and Fe(η5-C5H5)(η5-C4H4N) ("azaferrocene"). Azaferrocene arises from decarbonylation of Fe(η5-C5H5)(CO)2(η1-pyrrole) in cyclohexane.[76] This compound on boiling under reflux in benzene is converted to ferrocene.[77]
Because of the ease of substitution, many structurally unusual ferrocene derivatives have been prepared. For example, the penta(ferrocenyl)cyclopentadienyl ligand,[78] features a cyclopentadienyl anion derivatized with five ferrocene substituents.


In hexaferrocenylbenzene, C6[(η5-C5H4)Fe(η5-C5H5)]6, all six positions on a benzene molecule have ferrocenyl substituents (R).[79] X-ray diffraction analysis of this compound confirms that the cyclopentadienyl ligands are not co-planar with the benzene core but have alternating dihedral angles of +30° and −80°. Due to steric crowding the ferrocenyls are slightly bent with angles of 177° and have elongated C-Fe bonds. The quaternary cyclopentadienyl carbon atoms are also pyramidalized. Also, the benzene core has a chair conformation with dihedral angles of 14° and displays bond length alternation between 142.7 pm and 141.1 pm, both indications of steric crowding of the substituents.
The synthesis of hexaferrocenylbenzene has been reported using Negishi coupling of hexaiodidobenzene and diferrocenylzinc, using tris(dibenzylideneacetone)dipalladium(0) as catalyst, in tetrahydrofuran:[79]
The yield is only 4%, which is further evidence consistent with substantial steric crowding around the arene core.
Materials chemistry
[edit]
Ferrocene, a precursor to iron nanoparticles, can be used as a catalyst for the production of carbon nanotubes.[81] Vinylferrocene can be converted to (polyvinylferrocene, PVFc), a ferrocenyl version of polystyrene (the phenyl groups are replaced with ferrocenyl groups). Another polyferrocene which can be formed is poly(2-(methacryloyloxy)ethyl ferrocenecarboxylate), PFcMA. In addition to using organic polymer backbones, these pendant ferrocene units have been attached to inorganic backbones such as polysiloxanes, polyphosphazenes, and polyphosphinoboranes, (–PH(R)–BH2–)n, and the resulting materials exhibit unusual physical and electronic properties relating to the ferrocene / ferrocinium redox couple.[80] Both PVFc and PFcMA have been tethered onto silica wafers and the wettability measured when the polymer chains are uncharged and when the ferrocene moieties are oxidised to produce positively charged groups. The contact angle with water on the PFcMA-coated wafers was 70° smaller following oxidation, while in the case of PVFc the decrease was 30°, and the switching of wettability is reversible. In the PFcMA case, the effect of lengthening the chains and hence introducing more ferrocene groups is significantly larger reductions in the contact angle upon oxidation.[80][82]
See also
[edit]References
[edit]- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 1041. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ "Ferrocene (102-54-5)". ChemicalBook. Retrieved 3 February 2010.
- ^ "FERROCENE Material safety data sheet". ChemSrc.
- ^ Lide DR, ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton, Florida: CRC Press. p. 3.258. ISBN 0-8493-0486-5.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0205". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Ferrocene MSDS". ScienceLab. Archived from the original on 2015-12-12. Retrieved 2015-11-25.
- ^ a b c d e Werner H (June 2012). "At least 60 years of ferrocene: the discovery and rediscovery of the sandwich complexes". Angewandte Chemie. 51 (25): 6052–6058. doi:10.1002/anie.201201598. PMID 22573490.
- ^ a b c d Pauson PL (2001). "Ferrocene—how it all began". Journal of Organometallic Chemistry. 637–639: 3–6. doi:10.1016/S0022-328X(01)01126-3.
- ^ a b c Miller SA, Tebboth JA, Tremaine JF (1952). "114. Dicyclopentadienyliron". J. Chem. Soc.: 632–635. doi:10.1039/JR9520000632.
- ^ a b c Laszlo P, Hoffmann R (January 2000). "Ferrocene: Ironclad History or Rashomon Tale?". Angewandte Chemie. 39 (1): 123–124. doi:10.1002/(SICI)1521-3773(20000103)39:1<123::AID-ANIE123>3.0.CO;2-Z. PMID 10649350.
- ^ a b c Kealy TJ, Pauson PL (1951). "A New Type of Organo-Iron Compound". Nature. 168 (4285): 1039–1040. Bibcode:1951Natur.168.1039K. doi:10.1038/1681039b0. S2CID 4181383.
- ^ a b Federman Neto A, Pelegrino AC, Darin VA (2004). "Ferrocene: 50 Years of Transition Metal Organometallic Chemistry — From Organic and Inorganic to Supramolecular Chemistry (Abstract)". Trends in Organometallic Chemistry. 4. Research Trends: 147–169.
- ^ Werner H (2008). Landmarks in Organo-Transition Metal Chemistry: A Personal View. New York: Springer Science. pp. 161–63. ISBN 978-0-387-09847-0.
- ^ Wilkinson G, Rosenblum M, Whiting MC, Woodward RB (1952-03-24). "The structure of iron bis-cyclopentadienyl". J. Am. Chem. Soc. 74 (8): 2125–2126. doi:10.1021/ja01128a527.
{{cite journal}}: CS1 maint: year (link) - ^ Woodward, R. B.; Rosenblum, M.; Whiting, M. C. (1952-06-02). "A NEW AROMATIC SYSTEM". Journal of the American Chemical Society. 74 (13): 3458–3459. doi:10.1021/ja01133a543. ISSN 0002-7863.
- ^ Sutton, Mike (2023-09-27). "Fifty years since the ferrocene furore". Chemistry World. Retrieved 2024-11-02.
- ^ Fischer EO, Pfab W (1952). "Zur Kristallstruktur der Di-Cyclopentadienyl-Verbindungen des zweiwertigen Eisens, Kobalts und Nickels" [On the crystal structure of the bis-cyclopentadienyl compounds of divalent iron, cobalt and nickel]. Zeitschrift für Naturforschung B. 7 (7): 377–379. doi:10.1515/znb-1952-0701.
- ^ Okuda J (2016-12-28). "Ferrocene - 65 Years After". European Journal of Inorganic Chemistry. 2017 (2): 217–219. doi:10.1002/ejic.201601323. ISSN 1434-1948.
- ^ a b Eiland PF, Pepinsky R (1952). "X-ray Examination of Iron Biscyclopentadienyl". J. Am. Chem. Soc. 74 (19): 4971. doi:10.1021/ja01139a527.
- ^ Dunitz JD, Orgel LE (1953). "Bis-Cyclopentadienyl – A Molecular Sandwich". Nature. 171 (4342): 121–122. Bibcode:1953Natur.171..121D. doi:10.1038/171121a0. S2CID 4263761.
- ^ Dunitz JD, Orgel L, Rich A (1956). "The crystal structure of ferrocene". Acta Crystallogr. 9 (4): 373–375. doi:10.1107/S0365110X56001091.
- ^ Mingos DM (2001). "A Historical Perspective on Dewar's Landmark Contribution to Organometallic Chemistry". J. Organomet. Chem. 635 (1–2): 1–8. doi:10.1016/S0022-328X(01)01155-X.
- ^ Mehrotra RC, Singh A (2007). Organometallic Chemistry: A Unified Approach (2nd ed.). New Delhi: New Age International. pp. 261–67. ISBN 978-81-224-1258-1.
- ^ "The Nobel Prize in Chemistry 1973". Nobel Foundation. Retrieved 12 September 2010.
- ^ Seiler P, Dunitz JD (1982). "Low-temperature crystallization of orthorhombic ferrocene: structure analysis at 98 K". Acta Crystallographica Section B. 38 (6): 1741–1745. doi:10.1107/s0567740882007080. ISSN 0567-7408.
- ^ Haaland A, Nilsson JE (1968). "The Determination of Barriers to Internal Rotation by Means of Electron Diffraction. Ferrocene and Ruthenocene". Acta Chem. Scand. 22: 2653–2670. doi:10.3891/acta.chem.scand.22-2653.
- ^ Coriani S, Haaland A, Helgaker T, Jørgensen P (January 2006). "The equilibrium structure of ferrocene". ChemPhysChem. 7 (1): 245–249. doi:10.1002/cphc.200500339. PMID 16404766.
- ^ Katrusiak, Andrzej; Rusek, Michalina; Dušek, Michal; Petříček, Václav; Szafrański, Marek (2023-04-06). "Dipole-Moment Modulation in New Incommensurate Ferrocene". The Journal of Physical Chemistry Letters. 14 (13): 3111–3119. doi:10.1021/acs.jpclett.3c00215. ISSN 1948-7185. PMC 10084461. PMID 36951481.
- ^ Abel EW, Long NJ, Orrell KG, Osborne AG, Šik V (1991). "Dynamic NMR studies of ring rotation in substituted ferrocenes and ruthenocenes". J. Org. Chem. 403 (1–2): 195–208. doi:10.1016/0022-328X(91)83100-I.
- ^ Wang, Feng; Mohammadi, Narges; Best, Stephen P.; Appadoo, Dominique; Chantler, Christopher T. (2021). "Dominance of eclipsed ferrocene conformer in solutions revealed by the IR spectra between 400 and 500 cm-1". Radiation Physics and Chemistry. 188: 109590. Bibcode:2021RaPC..18809590W. doi:10.1016/j.radphyschem.2021.109590.
- ^ Wilkinson G, Pauson PL, Cotton FA (1954). "Bis-cyclopentadienyl Compounds of Nickel and Cobalt". J. Am. Chem. Soc. 76 (7): 1970–1974. doi:10.1021/ja01636a080.
- ^ Wilkinson G, Cotton FA (1959). "Cyclopentadienyl and Arene Metal Compounds". Progress in Inorganic Chemistry. Vol. 1. pp. 1–124. doi:10.1002/9780470166024.ch1. ISBN 978-0-470-16602-4.
- ^ a b Wilkinson G (1956). "Ferrocene". Organic Syntheses. 36: 31. doi:10.15227/orgsyn.036.0031.
- ^ Jolly WL (1970). The Synthesis and Characterization of Inorganic Compounds. New Jersey: Prentice-Hall. ISBN 9780138799328.
- ^ Wilkinson G, Cotton FA, Birmingham JM (1956). "On manganese cyclopentadienide and some chemical reactions of neutral bis-cyclopentadienyl metal compounds". J. Inorg. Nucl. Chem. 2 (2): 95–113. doi:10.1016/0022-1902(56)80004-3.
- ^ Solomons G, Craig F (2006). Organic Chemistry (9th ed.). USA: John Wiley & Sons.
- ^ Monte MJ, Santos LM, Fulem M, Fonseca JM, Sousa CA (2006). "New Static Apparatus and Vapor Pressure of Reference Materials: Naphthalene, Benzoic Acid, Benzophenone, and Ferrocene". Journal of Chemical & Engineering Data. 51 (2): 757. doi:10.1021/je050502y.
- ^ Fulem M, Růžička K, Červinka C, Rocha MA, Santos LM, Berg RF (2013). "Recommended vapor pressure and thermophysical data for ferrocene". Journal of Chemical Thermodynamics. 57: 530–540. doi:10.1016/j.jct.2012.07.023.
- ^ Bozak RE (1966). "Acetylation of Ferrocene: A Chromatography Experiment for Elementary Organic Laboratory". J. Chem. Educ. 43 (2): 73. Bibcode:1966JChEd..43...73B. doi:10.1021/ed043p73.
- ^ Rausch, M. D. (1963). "Metallocene Chemistry—A Decade of Progress". Canadian Journal of Chemistry. 41 (5): 1289–1314. doi:10.1139/v63-182.
- ^ Malischewski M, Seppelt K, Sutter J, Heinemann FW, Dittrich B, Meyer K (October 2017). "Protonation of Ferrocene: A Low-Temperature X-ray Diffraction Study of [Cp2 FeH](PF6 ) Reveals an Iron-Bound Hydrido Ligand". Angewandte Chemie. 56 (43): 13372–13376. doi:10.1002/anie.201704854. PMID 28834022.
- ^ Knox GR, Pauson PL, Willison D (1992). "Ferrocene derivatives. 27. Ferrocenyldimethylphosphine". Organometallics. 11 (8): 2930–2933. doi:10.1021/om00044a038.
- ^ Sollott GP, Mertwoy HE, Portnoy S, Snead JL (1963). "Unsymmetrical Tertiary Phosphines of Ferrocene by Friedel–Crafts Reactions. I. Ferrocenylphenylphosphines". J. Org. Chem. 28 (4): 1090–1092. doi:10.1021/jo01039a055.
- ^ St J Foreman MR, Alexandra MZ, Slawin AM, Woollins JD (1996). "2,4-Diferrocenyl-1,3-dithiadiphosphetane 2,4-disulfide; structure and reactions with catechols and [PtCl2(PR3)2](R = Et or Bun)". J. Chem. Soc., Dalton Trans. (18): 3653–3657. doi:10.1039/DT9960003653.
- ^ Busacca, Carl A.; Eriksson, Magnus C.; Haddad, Nizar; Han, Z. Steve; Lorenz, Jon C.; Qu, Bo; Zeng, Xingzhong; Senanayake, Chris H. (2013). "Practical Synthesis of Di-tert-Butyl-Phosphinoferrocene". Organic Syntheses. 90: 316. doi:10.15227/orgsyn.090.0316.
- ^ Cardona CM, Li W, Kaifer AE, Stockdale D, Bazan GC (May 2011). "Electrochemical considerations for determining absolute frontier orbital energy levels of conjugated polymers for solar cell applications". Advanced Materials. 23 (20): 2367–2371. Bibcode:2011AdM....23.2367C. doi:10.1002/adma.201004554. PMID 21462372. S2CID 40766788.
- ^ Vitaly V Pavlishchuk, Anthony W Addison (January 2000), "Conversion constants for redox potentials measured versus different reference electrodes in acetonitrile solutions at 25°C", Inorganica Chimica Acta (in German), vol. 298, no. 1, pp. 97–102, doi:10.1016/S0020-1693(99)00407-7, retrieved 2022-07-26
- ^ Connelly NG, Geiger WE (March 1996). "Chemical Redox Agents for Organometallic Chemistry". Chemical Reviews. 96 (2): 877–910. doi:10.1021/cr940053x. PMID 11848774.
- ^ Sirbu D, Turta C, Gibson EA, Benniston AC (September 2015). "The ferrocene effect: enhanced electrocatalytic hydrogen production using meso-tetraferrocenyl porphyrin palladium(II) and copper(II) complexes". Dalton Transactions. 44 (33): 14646–14655. doi:10.1039/C5DT02191J. PMID 26213204.
- ^ Lennox AJ, Nutting JE, Stahl SS (January 2018). "Selective electrochemical generation of benzylic radicals enabled by ferrocene-based electron-transfer mediators". Chemical Science. 9 (2): 356–361. doi:10.1039/C7SC04032F. PMC 5909123. PMID 29732109.
- ^ Dannenberg JJ, Richards JH (1965-04-01). "Photosensitization by Ferrocene. Photochemistry of Higher Electronic Excited States". Journal of the American Chemical Society. 87 (7): 1626–1627. doi:10.1021/ja01085a048. ISSN 0002-7863.
- ^ Sirbu D, Turta C, Benniston AC, Abou-Chahine F, Lemmetyinen H, Tkachenko NV, et al. (2014-05-23). "Synthesis and properties of a meso- tris–ferrocene appended zinc(II) porphyrin and a critical evaluation of its dye sensitised solar cell (DSSC) performance". RSC Advances. 4 (43): 22733–22742. Bibcode:2014RSCAd...422733S. doi:10.1039/C4RA03105A. ISSN 2046-2069.
- ^ Malischewski M, Adelhardt M, Sutter J, Meyer K, Seppelt K (August 2016). "Isolation and structural and electronic characterization of salts of the decamethylferrocene dication". Science. 353 (6300): 678–682. Bibcode:2016Sci...353..678M. doi:10.1126/science.aaf6362. PMID 27516596. S2CID 43385610.
- ^ Ruble JC, Latham HA, Fu GC (1997). "Effective Kinetic Resolution of Secondary Alcohols with a Planar-Chiral Analogue of 4-(dimethylamino)pyridine. Use of the Fe(C5Ph5) Group in Asymmetric Catalysis". J. Am. Chem. Soc. 119 (6): 1492–1493. doi:10.1021/ja963835b.
- ^ Atkinson RC, Gibson VC, Long NJ (June 2004). "The syntheses and catalytic applications of unsymmetrical ferrocene ligands". Chemical Society Reviews. 33 (5): 313–328. doi:10.1039/B316819K. PMID 15272371.
- ^ a b Blaser HU (2002). "Solvias Josiphos ligands: From discovery to technical applications". Topics in Catalysis. 19: 3–16. doi:10.1023/a:1013832630565. S2CID 95738043.
- ^ Zhou QL (2011). Privileged Chiral Ligands and Catalysts. Weinheim: Wiley-VCH Verlag. ISBN 978-3-527-63521-4.
- ^ a b Stepnicka P (2008). Ferrocenes: Ligands, Materials and Biomolecules. Hoboken, NJ: J. Wiley. ISBN 978-0-470-03585-6.
- ^ Bennett J (26 November 2004). Application of fuel additives (PDF). International Conference on Automotive Technology. Istanbul. Archived from the original (PDF) on 2006-05-05.
- ^ US 4104036, Chao TS, "Iron-containing motor fuel compositions and method for using same", published 1978-08-01, assigned to Atlantic Richfield Co
- ^ US 3598850, Dewey FM, "Ferrocene Polyglycols", issued Aug. 10, 1971, assigned to U.S. Air Force.
- ^ US 3927992, Kerley RV, "Coal Combustion Process and Composition", issued December 23, 1975, assigned to Ethyl Corporation.
- ^ van Staveren DR, Metzler-Nolte N (December 2004). "Bioorganometallic chemistry of ferrocene". Chemical Reviews. 104 (12): 5931–5985. doi:10.1021/cr0101510. PMID 15584693.
- ^ Ong YC, Gasser G (December 2020). "Organometallic compounds in drug discovery: Past, present and future" (PDF). Drug Discovery Today: Technologies. 37: 117–124. doi:10.1016/j.ddtec.2019.06.001. PMID 34895650. S2CID 198268304.
- ^ Biot C, Nosten F, Fraisse L, Ter-Minassian D, Khalife J, Dive D (August 2011). "The antimalarial ferroquine: from bench to clinic". Parasite. 18 (3): 207–214. doi:10.1051/parasite/2011183207. PMC 3671469. PMID 21894260.

- ^ Roux C, Biot C (April 2012). "Ferrocene-based antimalarials". Future Medicinal Chemistry. 4 (6): 783–797. doi:10.4155/fmc.12.26. PMID 22530641.
- ^ Wani WA, Jameel E, Baig U, Mumtazuddin S, Hun LT (August 2015). "Ferroquine and its derivatives: new generation of antimalarial agents". European Journal of Medicinal Chemistry. 101: 534–551. doi:10.1016/j.ejmech.2015.07.009. PMC 7115395. PMID 26188909.
- ^ Adoke Y, Zoleko-Manego R, Ouoba S, Tiono AB, Kaguthi G, Bonzela JE, et al. (May 2021). "A randomized, double-blind, phase 2b study to investigate the efficacy, safety, tolerability and pharmacokinetics of a single-dose regimen of ferroquine with artefenomel in adults and children with uncomplicated Plasmodium falciparum malaria". Malaria Journal. 20 (1): 222. doi:10.1186/s12936-021-03749-4. PMC 8135182. PMID 34011358.
- ^ Gu H, Mu S, Qiu G, Liu X, Zhang L, Yuan Y, Astruc D (June 2018). "Redox-stimuli-responsive drug delivery systems with supramolecular ferrocenyl-containing polymers for controlled release". Coordination Chemistry Reviews. 364: 51–85. doi:10.1016/j.ccr.2018.03.013. ISSN 0010-8545. S2CID 103022297.
- ^ Ornelas C (2011). "Application of ferrocene and its derivatives in cancer research". New Journal of Chemistry. 35 (10): 1973. doi:10.1039/c1nj20172g. S2CID 56521492.
- ^ Babin VN, Belousov YA, Borisov VI, Gumenyuk VV, Nekrasov YS, Ostrovskaya LA, et al. (2014). "Ferrocenes as potential anticancer drugs. Facts and hypotheses". Russ. Chem. Bull. 63 (11): 2405–2422. doi:10.1007/s11172-014-0756-7. S2CID 94618726.
- ^ US 9738673, Yong J, Lu C, "Ferrocene Derivative, Preparation Method and Use Thereof.", issued August 22, 2017, assigned to Xiamen Institute of Rare Earth Materials.
- ^ a b Top S, Vessières A, Leclercq G, Quivy J, Tang J, Vaissermann J, et al. (November 2003). "Synthesis, biochemical properties and molecular modelling studies of organometallic specific estrogen receptor modulators (SERMs), the ferrocifens and hydroxyferrocifens: evidence for an antiproliferative effect of hydroxyferrocifens on both hormone-dependent and hormone-independent breast cancer cell lines". Chemistry: A European Journal. 9 (21): 5223–5236. doi:10.1002/chem.200305024. PMID 14613131.
- ^ Dagani R (16 September 2002). "The Bio Side of Organometallics". Chemical and Engineering News. 80 (37): 23–29. doi:10.1021/cen-v080n037.p023.
- ^ "Ferrocene Burn Rate Catalyst". www.rocketmotorparts.com. Retrieved 2020-01-13.
- ^ Zakrzewski J, Giannotti C (1990). "An improved photochemical synthesis of azaferrocene". J. Organomet. Chem. 388 (1–2): 175–179. doi:10.1016/0022-328X(90)85359-7.
- ^ Efraty A, Jubran N, Goldman A (1982). "Chemistry of some η5-pyrrolyl- and η1-N-pyrrolyliron complexes". Inorg. Chem. 21 (3): 868–873. doi:10.1021/ic00133a006.
- ^ Yu Y, Bond AD, Leonard PW, Vollhardt KP, Whitener GD (March 2006). "Syntheses, structures, and reactivity of radial oligocyclopentadienyl metal complexes: penta(ferrocenyl)cyclopentadienyl and congeners". Angewandte Chemie. 45 (11): 1794–1799. doi:10.1002/anie.200504047. PMID 16470902.
- ^ a b Yu Y, Bond AD, Leonard PW, Lorenz UJ, Timofeeva TV, Vollhardt KP, et al. (June 2006). "Hexaferrocenylbenzene". Chemical Communications (24): 2572–2574. doi:10.1039/b604844g. PMID 16779481.
- ^ a b c Pietschnig R (October 2016). "Polymers with pendant ferrocenes". Chemical Society Reviews. 45 (19): 5216–5231. doi:10.1039/C6CS00196C. PMID 27156979.
- ^ Conroy D, Moisala A, Cardoso S, Windle A, Davidson J (2010). "Carbon nanotube reactor: Ferrocene decomposition, iron particle growth, nanotube aggregation and scale-up". Chem. Eng. Sci. 65 (10): 2965–2977. doi:10.1016/j.ces.2010.01.019.
- ^ Elbert J, Gallei M, Rüttiger C, Brunsen A, Didzoleit H, Stühn B, Rehahn M (2013). "Ferrocene Polymers for Switchable Surface Wettability". Organometallics. 32 (20): 5873–5878. doi:10.1021/om400468p.
External links
[edit]- Ferrocene at The Periodic Table of Videos (University of Nottingham)
- NIOSH Pocket Guide to Chemical Hazards (Centers for Disease Control and Prevention)







